Photochemistry provides a pathway to swap the oxygen atom in oxetane rings with nitrogen, carbon or sulfur at a single stroke. The resulting structures, such as azetidines and thietanes, are usually unattainable with traditional reactions and yet attractive to medicinal chemists. ‘This paper pushes skeletal editing beyond aromatic scaffolds, which represents a stimulating discovery,’ says Karen de la Vega, an expert in late-stage functionalisation at the Institute of Chemical Research of Catalonia, Spain, who wasn’t involved in the study.
‘Oxetane is a very versatile motif in medicinal chemistry,’ says Jianwei Sun, an expert in bioactive compounds at the Hong Kong University of Science and Technology. In pharmaceuticals, carbonyls are often replaced by more stable compounds with similar biological activity – known as bioisosteres – such as oxetanes. The direct transformation of oxetane into other four-membered heterocycles could create many opportunities in drug design and development, says Sun.
Additionally, atom-swapping strategies provide a greener alternative to traditional routes. ‘The retrosynthesis of four-membered cyclic compounds largely relies on deconstructing the ring into simpler starting materials, which require separate preparations and numerous steps,’ says lead author Ming Joo Koh from the University of Singapore. This typically takes a long time and produces unwanted waste.
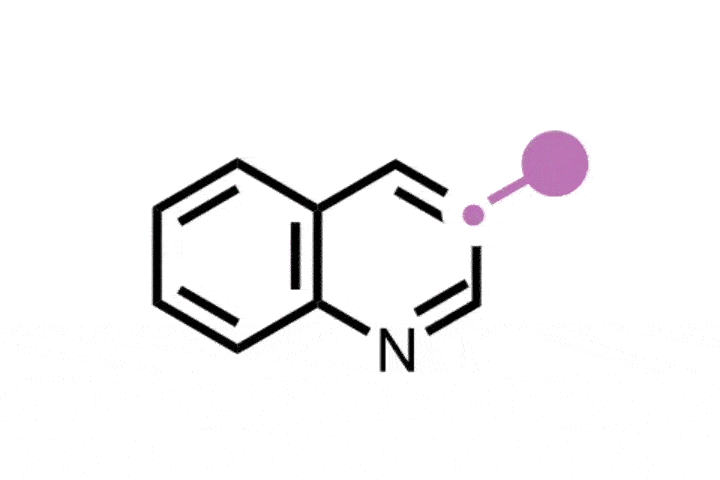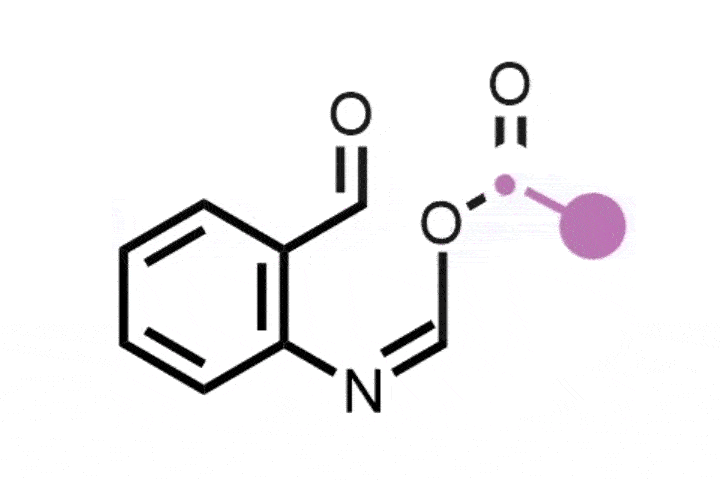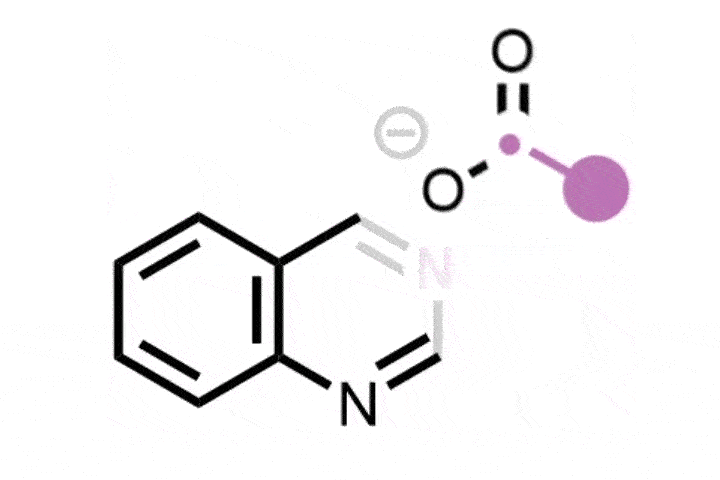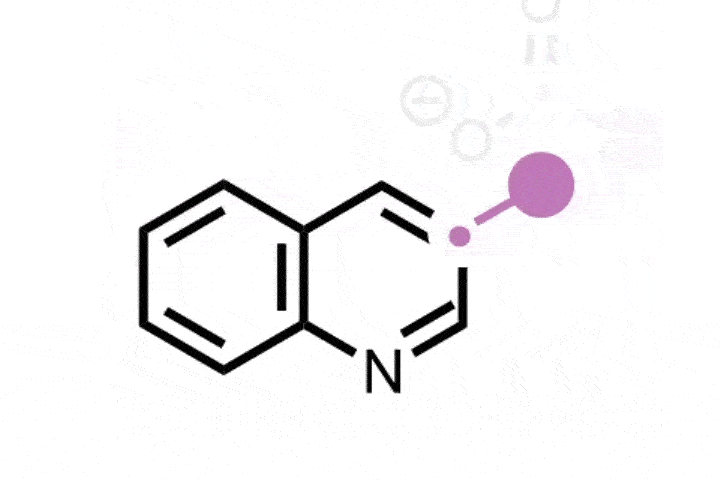
The researchers envisioned a one-pot, two-step process to replace the oxygen in oxetanes by other atoms. They started by exposing oxetane to blue light in the presence of a ruthenium photocatalyst, which led to the formation of an open dibromide intermediate, ‘unique to this approach’, according to Koh. Sun also describes it as ‘unusual’, as the opening of the oxetane should result in scaffolds with oxygen moieties. Photochemistry creates the perfect conditions to carry out the reaction under mild conditions, compared with other oxetane openings, explains Sun. Additionally, the dibromide ‘is easily converted into diverse strained rings’. The subsequent step simply combines the dibromide with different nucleophiles such as sulfides, amines and alkylating agents – to insert sulfur, nitrogen and carbon respectively.
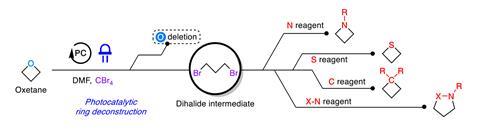
It is ‘especially exciting’ how skeletal editing extends the value and versatility of oxetanes, says De la Vega. ‘The direct access to other ring systems enables late-stage adjustments to solubility and metabolic stability without restarting a synthetic strategy from scratch,’ she adds. Whereas the traditional tailoring of saturated heterocycles is long and substrate-specific, ‘atom swapping is elegant and efficient’. The new approach ‘dramatically reduces the reaction steps … using a single precursor and a range of nucleophiles to access different products in one pot’, says De la Vega. Moreover, this process permits swapping the oxygen in oxetane for several atoms at once. ‘Strictly, skeletal editing refers to single-atom manipulations, however this further demonstrates the versatility of the reaction for greater structural reshaping beyond traditional transmutations,’ she explains.
Because of their rigidity and small size, saturated rings possess some desirable properties for drug discovery, explains Koh. His atom swapping strategy substantially simplifies the synthesis of complex structures, which would otherwise require tortuous routes. To demonstrate this, the team swapped the oxygen atom with sulfur in a pharmaceutical candidate – a phosphodiesterase-4 inhibitor under investigation for the treatment of inflammatory diseases. ‘The thietane bioisostere was reported in a patent as three times more potent,’ says Koh. ‘Our approach offers a straightforward [strategy towards] libraries of drug analogues without having to start [substances] from scratch,’ he adds.
The substitution of oxygen with sulfur, nitrogen or methylene groups also serves as an easy way to adjust the solubility, stability and strength of a drug. ‘Now, this atom-swapping approach provides a powerful shortcut to access analogues with potentially improved properties, which is tremendously attractive in medicinal chemistry,’ adds de la Vega.
References
Y-Q Zhang et al, Nature, 2025, DOI: 10.1038/s41586-025-09723-3











No comments yet