Compound bearing 10 nitro groups could cut dangerous emissions from rocket launches
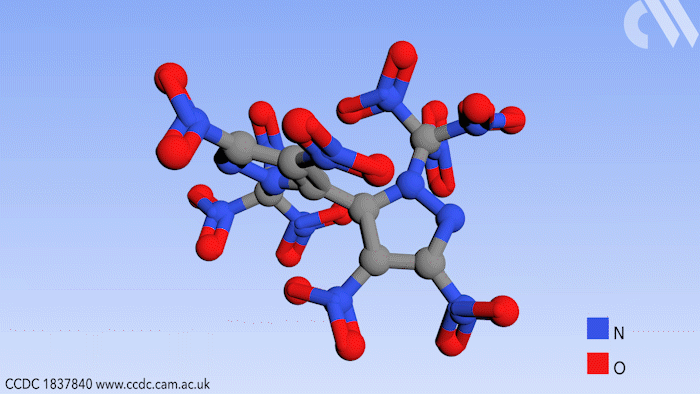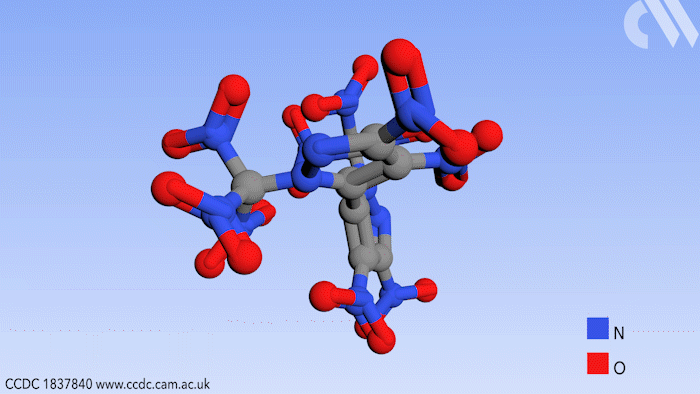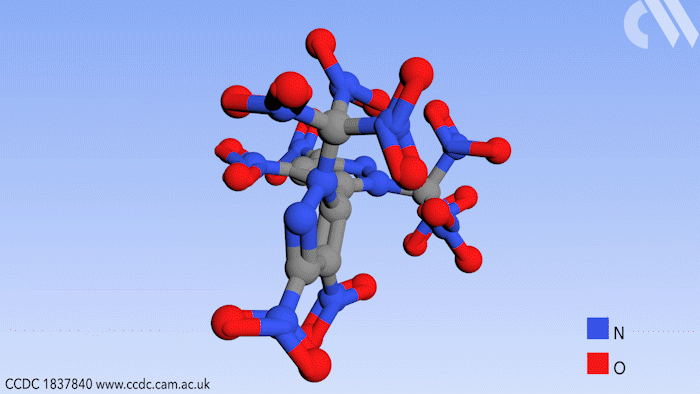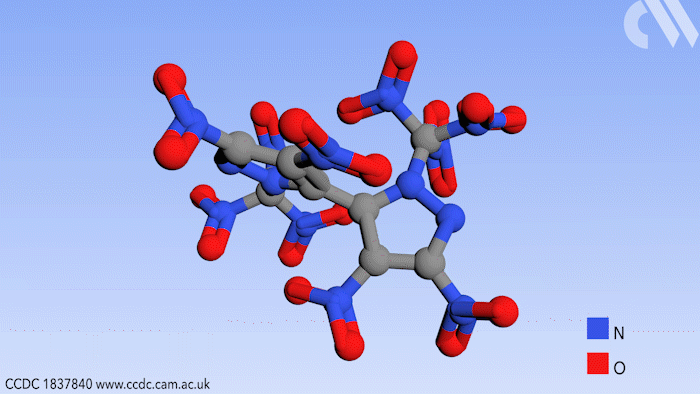
Researchers in Russia have prepared a new high energy compound for use in environmentally friendly rocket fuels. Use of this compound could effectively eliminate a hazardous byproduct from the process of jet propulsion.
As safety concerns dictate that rocket fuels be kept away from air, a key component of their formulation is an oxidiser that can serve as an oxygen source to assist the combustion reaction that propels the rocket. Ammonium perchlorate is a widely used oxidiser in rockets and other pyrotechnics. However, a major combustion product of this is hydrochloric acid, HCl, which can have an adverse effect on the ozone layer and cause acid rain. Developing an oxidiser that does not contain chlorine is therefore a worthwhile goal.
Now a study, conducted in the group of Aleksei Sheremetev at the Russian Academy of Sciences in Moscow, shows a synthetic route to a bipyrazole molecule that contains no fewer than 10 nitro groups, an important functional group in explosive materials. More importantly, it contains no chlorine, thereby eliminating HCl as a possible byproduct.
Beginning from a starting material with four nitro groups, a further six were added in two steps by first introducing ketone substituents to the pyrazole rings that can react further with a mixture of nitric acid and sulfuric acid. This replaces the ketones with trinitromethyl groups to form the final product.
‘From an academic point of view, the researchers have to be congratulated on their finding,’ says Thomas Klapoetke, an expert in energetic materials based at the Ludwig-Maximillian University of Munich in Germany. However, he remains sceptical about its feasibility for practical use.
Although the molecule shows resistance to light and moisture and has a high density that would make it attractive for rocket fuels, its relatively low decomposition temperature of 125°C could prove to be a significant barrier. Klapoetke adds ‘Trinitromethyl compounds are always liable to low decomposition temperatures and degradation reactions with the binder system. The researchers should in future also measure the long term stability of a formulation of [the compound] with a real binder.’
References
This article is free to access until 19 September 2018
I L Dalinger et al, J. Mater. Chem. A, 2018 DOI: 10.1039/c8ta05179h








No comments yet