Introducing tetranitratoethane – it’s bursting with oxygen
Scientists in Germany have made tetranitratoethane (C2H2N4O12), a solid oxidiser with one of the highest oxygen contents ever synthesised. This research is part of an international search for new oxidisers to replace toxic ammonium perchlorate (NH4ClO4).
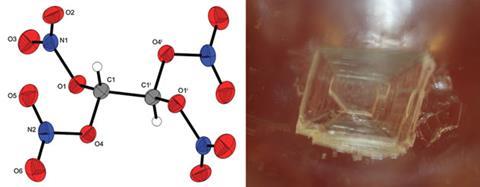
Oxidisers are materials that form molecules of oxygen when burned, in addition to regular combustion products H2O, N2, CO and CO2, and are described as having a positive oxygen balance. Tetranitratoethane, created by Thomas Klapötke and colleagues at the Ludwig-Maximilian University of Munich, has a higher oxygen content than other well-known solid oxidisers, and represents one of only a very few compounds where two or more oxygen-rich nitro (–NO2) groups are bonded to the same carbon atom.
With an oxygen content of 70.1% and a greater oxygen balance than ammonium perchlorate, tetranitratoethane is very sensitive to friction and impact, similar to nitroglycerine. Calculations of specific impulse from combustion, relevant to use as rocket fuel, indicate that tetranitratoethane theoretically performs better than ammonium perchlorate mixtures, amongst others.
However, although tetranitratoethane carries one of the highest oxygen contents ever synthesised, its low thermal stability and high sensitivity will likely prevent any immediate practical applications.
References
This article is free to access until 29 February 2016
D Fischer, T M Klapötke and J Stierstorfer, Chem. Commun., 2016, 52, 916 (DOI: 10.1039/c5cc09010e)











No comments yet