The first neutral nitrogen molecule – other than dinitrogen – has been isolated and characterised by researchers in Germany. The molecule, the most energetic ever synthesised, is effectively stable at liquid nitrogen temperatures, which could make it attractive as an energy storage material.
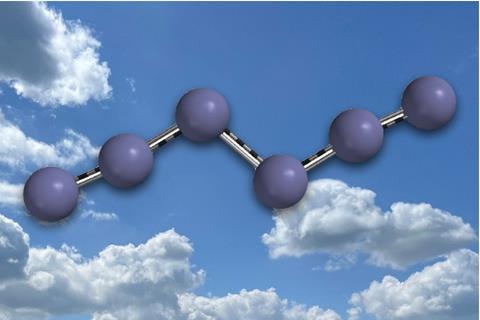
The dinitrogen molecule, which was first identified in the late 18th century, owes its extreme stability to its triple bond. Therefore, whereas atoms like carbon can exist in multiple allotropes, no other stable neutral pure nitrogen molecule has previously been identified, although exotic polymorphs exist at extremely high pressures. The azide radical was identified in 1956 and a tetranitrogen complex was glimpsed in mass spectra in 2002, but its structure remains undetermined.2 While the dinitrogen molecule is low in energy, other nitrogen-containing molecules such as trinitrotoluene (TNT) and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane, octogen (HMX) are the world’s most powerful non-nuclear explosives.
This work is spectacular and, in my opinion, is worthy of a Nobel prize
Karl Christe, polynitrogen pioneer
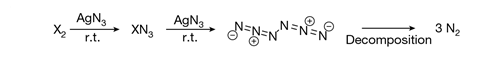
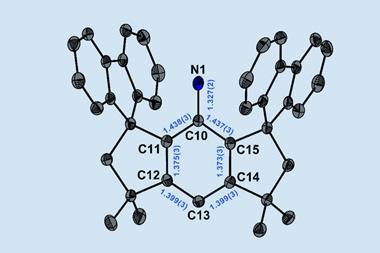
In the new work, organic chemists Weiyu Qian, Artur Mardyukov and Peter Schreiner at Justus Liebig University Giessen calculated that a hexanitrogen molecule could be relatively long lived because it contained no discernible dinitrogen units. They synthesised the molecule by flowing chlorine gas through solid silver azide under reduced pressure. Silver azide is an excellent reagent for the synthesis of both halogen azides and polyazides. The chlorine therefore reacted with the silver azide to produce chloroazide, which reacted further with more silver azide to produce silver chloride and hexanitrogen. The weakest point of the structure – the ‘Achilles heel’ – is between the nitrogen atoms next to the bond between the two azide units. This increases the molecule’s lifetime at room temperature to around 36 milliseconds – long enough for it to be trapped and cooled to liquid nitrogen temperatures, where the researchers calculate its half life to be over 100 years.
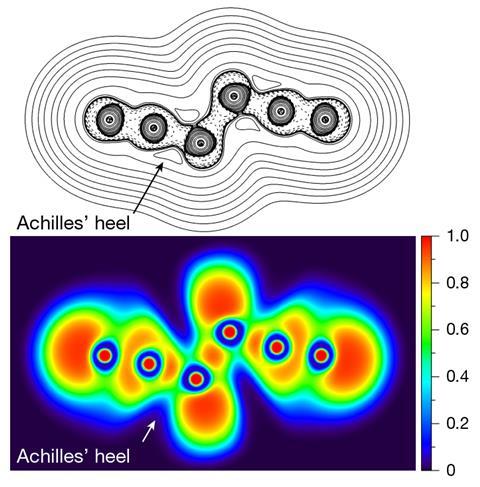
‘[This] work is truly outstanding,’ says Karl Christe at the University of Southern California, who pioneered early work on polynitrogen compounds. ’Compared to carbon chemistry, where two Nobel prizes were awarded for the discovery of two new carbon allotropes (buckyballs and graphene), the experimental observation of a neutral molecular nitrogen allotrope is orders of magnitude more difficult. The carbon allotropes are only a few kcal/mol higher in energy than graphite and are stabilised by huge activation barriers. In the case of the neutral molecular nitrogen allotrope, N6, the activation barriers are relatively small (26 and 15kcal/mol) and the energy difference, relative to N2, is huge (185kcal/mol). This work is spectacular and, in my opinion, is worthy of a Nobel prize.’
When hexanitrogen does break down, it releases double the energy per unit mass of HMX – currently the most powerful chemical explosive known. Unlike some explosives that can leave residual pollutants such as nitrates, the only product is dinitrogen. Schreiner suggests ‘it would be the most useful rocket fuel on the planet’. ‘N6 would not burn with a flame: it’s just a burst of energy that generates a large volume [of gas] – so a lot of thrust, and it is non-corrosive,’ he explains. ‘One of the big problems [for rockets] right now is corrosion with hydrazine and other fuels.’
The researchers are now seeking to synthesise even larger molecules based on the principles they have developed. ‘We believe N10 is feasible,’ says Schreiner. ‘Whether it can actually be made is a technical question, but we’re going to give it a good shot.’
‘I would say any time a researcher can establish the existence of a previously unknown allotrope of one of the six biogenic elements, rewriting the textbook chapters on what we know about nitrogen, that’s a pretty big deal,’ says synthetic chemist Christopher Cummins of the Massachusetts Institute of Technology in the US. ‘When it comes to high energy density materials, one of the attributes people always look for is what is the nitrogen content, and in this case it’s 100%… What does that say about what other wealth of compounds are out there waiting to be discovered if we’ve only just begun to explore this region of chemical space?’
Philipp Wagner at the University of Tübingen in Germany agrees. ‘I think the most remarkable thing is that they made this molecule at all,’ he says. ‘Whether it will have any applications or not remains to be seen.’
Karl Christe’s comment was added on 13 June 2025. The third paragraph passage on the weak point of the hexanitrogen molecule was corrected on 24 July 2025.
References
1 W Qian, A Mardyukov and PR Schreiner, Nature, 2025, 642, 356 (DOI: 10.1038/s41586-025-09032-9)
2 F Cacace, G de Petris and A Troiani, Science, 2002, 295, 480 (DOI: 10.1126/science.1067681)















No comments yet