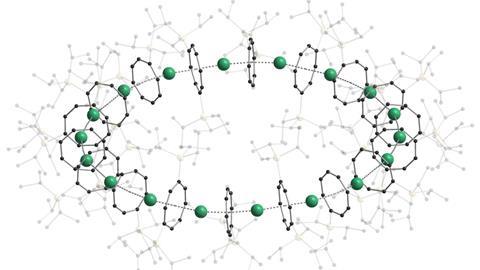
A new class of cyclic compounds features repeating units of sandwich complexes in closed, circular rings. The German research team that discovered them hope that ‘cyclocenes’ will open the door to new functional organometallic materials.
The isolation of the very first sandwich complex, ferrocene, was a major milestone for organometallic chemistry. Now the team led by Peter Roesky at the Karlsruhe Institute of Technology has synthesised cyclocene compounds containing up to 18 metallocene sandwich units.
The cyclocenes contain cyclooctatetraene (COT) ligands that have two bulky silane groups attached to them. These COT ligands sandwich the metal centres – either strontium, samarium, europium or ytterbium depending on the cyclocene formed. 18-unit cyclocenes are formed in the cases of strontium, samarium and europium, whereas using ytterbium leads to a four-unit structure. This is due to ytterbium’s smaller ionic radius, which means that it is more likely to form a Lewis acid-base adduct. While the other cyclocenes lose all solvent ligands, the ytterbium units retain a THF adduct. This extra THF sterically hinders the formation of a larger ring.
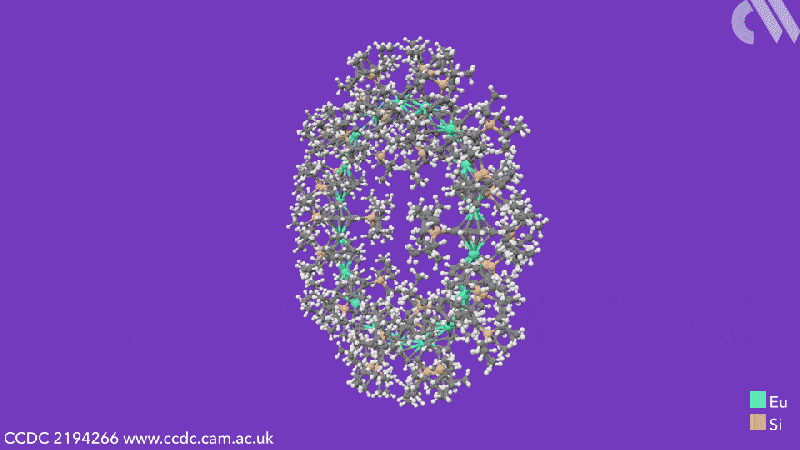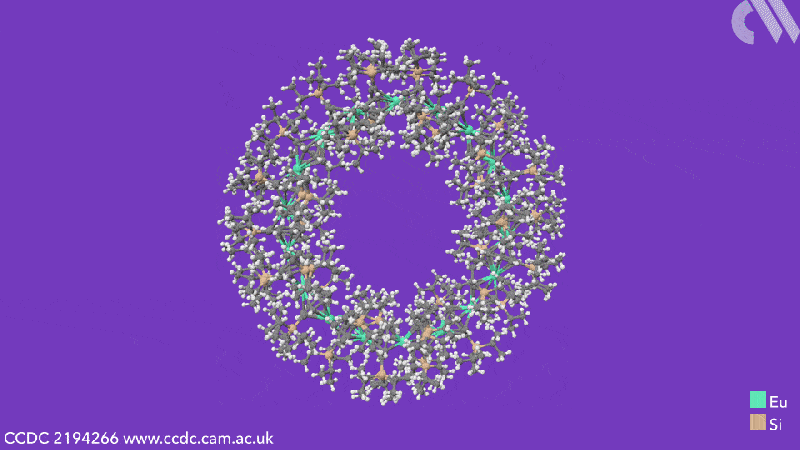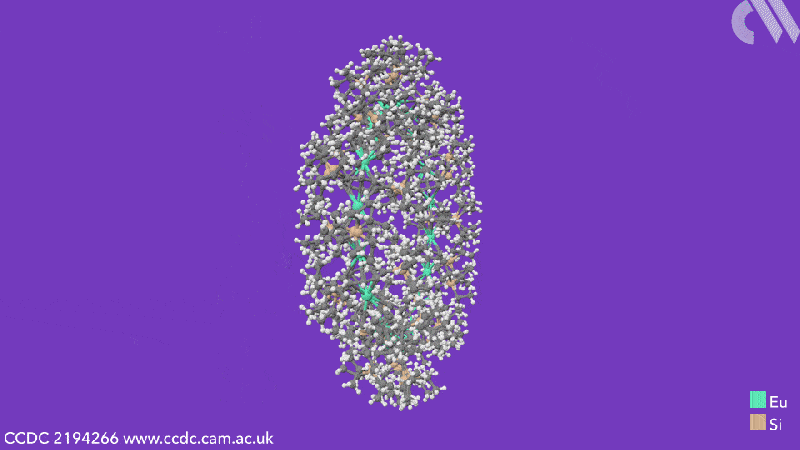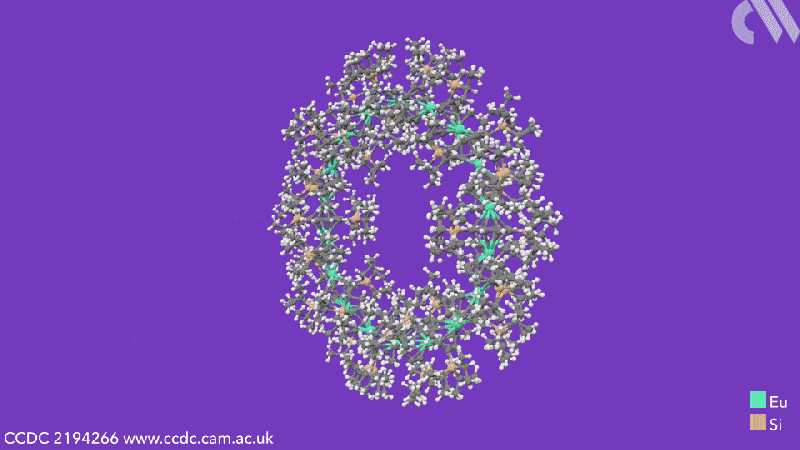
Computational analysis highlights the energy gain from ring closure as the reason the cyclic structures form, while the pronounced bending between the metals and their ligands is due to the steric demands from the COT substituents. The researchers believe that this will enable them to control the size of future cyclocenes by tailoring the ligand systems.
Correction: This story was updated on 14 August 2023, as a previous version incorrectly stated that the cyclocene compounds featured cyclobutadiene rather than cyclooctatetraene ligands.
References
L Münzfeld et al, Nature, 2023, DOI: 10.1038/s41586-023-06192-4














No comments yet