Rechargeable aqueous aluminium batteries must overcome some fundamental problems before they can hit the market, new research shows.
Having emerged as a more sustainable alternative to lithium-based batteries, aqueous aluminium batteries have been shown to have a high theoretical capacity. Moreover, aluminium’s inherent safety and non-toxicity have added to researchers’ excitement about the element featuring in energy storage devices.
On a fundamental level, the high Lewis acidity of the trivalent Al(iii) ion has hampered the development of aluminium batteries. Recent reports have proposed using aluminium trifluoromethanesulfonate in aqueous media (Al(OTf)3-H2O), which demonstrates a high cycling ability. However, details concerning the tightly bound trihydrate ligands, the resulting solvation structure, and its impact on the performance of these batteries were either unknown or ambiguous.
Now, a team surrounding Kang Xu, of Devcom Army Research Laboratory, has studied the solvation of aluminium electrolytes. The team’s results suggest that the solvation interactions of trivalent aluminium pose fundamental challenges to the feasibility of aqueous aluminium batteries.
‘Working independently, we likely would have put forth an incomplete or misleading description of mechanisms ascribed to the aqueous Al(OTf)3 electrolyte,’ explains Devcom team member Glenn Pastel. Collaborators at Sandia National Laboratory and Hunter College of the City University of New York, who used advanced characterisation techniques to explore the solvation, transport and reactivity of the aqueous Al(OTf)3 electrolyte, were therefore crucial to the study.
Through a combination of single-crystal x-ray diffraction and nuclear magnetic resonance spectroscopy (NMR), the team was able to probe the solvation structure of Al(OTf)3. In the solid state, the structure corresponds to Al(OTf)3·9H2O and surprisingly, no OTf- anions form contact pairs with Al(iii) in the first solvation sheath.
For additional insight into the liquid solvation structure of potential electrolytes, NMR spectroscopy revealed that Al(iii) coordinates closely to the oxygen atoms from the six water ligands in the first solvation sheath. These results agree closely with the solid-state structure the team obtained. However, unlike in the solid state, solutions may act as Brønsted–Lowry acids. As a result, efforts to improve cathodic stability by leveraging higher salt concentrations of Al(OTf)3 are counterproductive and do not provide a sufficient stability window for aluminium stripping and plating.
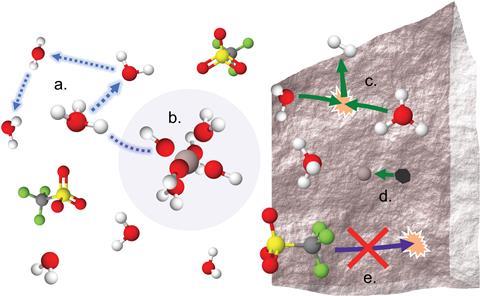
Furthermore, Pourbaix diagram predictions, which plot the equilibrium potential of electrochemical reactions against pH, suggest that many of the substrates previously considered for aqueous aluminium batteries are susceptible to corrosion in the acidic (Al(OTf)3-H2O) solutions, further hindering performance.
Whether aqueous aluminium batteries remain a feasible alternative to lithium-ion batteries is unclear. Aluminium metal anodes are likely to continue to be challenging to pair with aqueous electrolytes, given that the reduction potential of aluminium sits outside the stability window of water. The team envision that their work will help redirect efforts related to aqueous aluminium electrolytes to address the challenges related to parasitic reactions and solvation in other multivalent and aqueous electrolytes.
‘The search and investigation of new types and more sustainable electrochemical storage systems are fundamental for the transition to a green energy economy, and aluminium has exciting characteristics as an energy carrier,’ comments Giuseppe Antonio Elia, who studies batteries at the Polytechnic University of Turin in Italy. ‘However, we need to be very careful in our search, especially when new chemistries are proposed.’
References
G R Pastel et al, Energy Environ. Sci., 2022, DOI: 10.1039/d2ee00134a









1 Reader's comment