
Nanodiamonds – important for wide-ranging applications, including biomedical imaging, drug delivery, quantum computing and sensors – have now been made from petroleum-derived adamantane crystals by firing high-energy electrons at them. The synthesis, which was discovered by chance, creates defect-free nanodiamonds under ultra-low-temperatures in a vacuum without catalysts, additives or a support medium and could offer a quick, safer and low-cost alternative to existing high pressure and high temperature methods.
Adamantane is a naturally occurring crystalline hydrocarbon isolated from petroleum whose structure closely resembles diamond, both having tetrahedral symmetric carbon skeletons with their carbon atoms arranged in the same spacial pattern. Owing to their similarity, it was thought that, in theory, the C–H bonds of adamantane could be precisely cut, or activated, to form new C–C bonds and thus convert it to diamond.
‘The real problem was that no one thought it feasible,’ says Eiichi Nakamura at the University of Tokyo, Japan. Now, however, Nakamura’s lab has stumbled upon a way to do it, by irradiating adamantane with high-energy electrons. ‘Our synthesis is a true bottom-up synthesis, where adamantane molecules are assembled one-by-one three-dimensionally to form spherical single crystalline nanodiamonds of narrow size distribution,’ he explains.
In conventional, top-down syntheses, nanodiamonds are obtained by converting carbon sources under extreme conditions – pressures of tens of gigapascals and temperatures of thousands of kelvin – to produce thermodynamically stable diamond, or chemical vapour deposition techniques where the end product is unstable.
The team inadvertently made the discovery when following on from an unrelated study that had devised a way to use electron diffraction to measure the entropy of melting crystals. They were particularly intrigued by the observation of an extremely small entropy disorder in adamantane, which was comparable to that of gold crystals.
Curious about this, Nakamura’s student Jiarui Fu set out to explore the electron diffraction more closely, carefully firing electrons for a long time at an adamantane crystal that was already disordered and not supposed to show any diffraction peaks. But unexpectedly, Fu saw the gradual appearance of diffraction rings. ‘It became immediately apparent for him that the rings were due to nanoscale diamonds,’ says Nakamura.
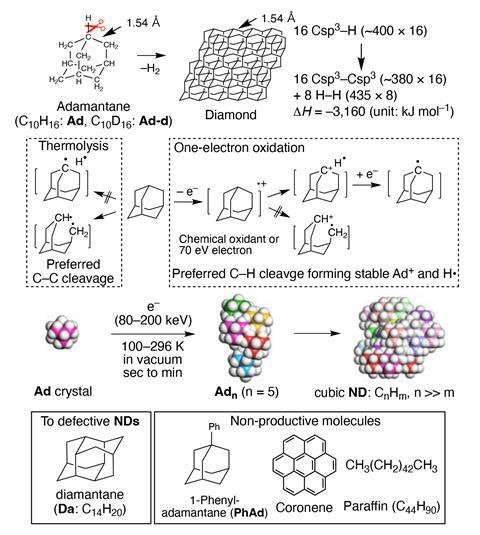
Further experiments honed the process, demonstrating that electron irradiation of adamatane crystals at between 80 and 200 kiloelectron volts in a vacuum at 100K for tens of seconds produced defect-free nanodiamonds 2–4nm in diameter, while giving off hydrogen gas. Time-resolved transmission electron microscopy revealed the initial formation of adamantane oligomers and their transformation into spherical nanodiamonds. Meanwhile, other hydrocarbons that were tested didn’t form nanodiamonds.
‘The findings open a new paradigm for understanding and controlling chemistry in the fields of electron lithography and surface engineering,’ says Nakamura. One promising application, he says, is the synthesis of doped quantum dots, essential for the construction of quantum computers and sensors. ‘By the use of the existing electron lithography setup, the method will be readily amenable for the construction of a nanodiamond array at the nanoscale.’
‘This novel approach capitalises on the unique property of diamondoids, which undergo single-electron oxidation to generate a radical cation that loses a hydrogen atom, leaving behind a radical that subsequently initiates subsequent reactions with additional starting materials to form fused diamond particles,’ comments Peter Schreiner who studies nanodiamonds at the University of Giessen, Germany. ‘As adamantane is readily accessible and electron-irradiation scalable, this method promises a new, viable diamond particle synthesis.’
References
J Fu, T Nakamuro and E Nakamura, Science, 2025, DOI: 10.1126/science.adw2025











No comments yet