A simplified experimental approach for studying liquid metallic hydrogen has been developed by an international research team.1 The scientists claim they’ve turned plastics into hydrogen and carbon using an intense laser beam that produces crushing pressures and high temperatures that transforms these elements into diamonds and a liquid metal. The new results could have important applications in planetary science and materials synthesis.
’We’ve previously shown how plastic forms diamonds at high pressures2 and now we’re trying to understand what’s happening to the hydrogen at the same time,’ says Nicholas Hartley at SLAC National Accelerator Laboratory in the US. ‘Hydrogen at the kind of pressures we’re reaching behaves like a metal, and such metallic hydrogen forms the interior of Jupiter and Saturn, where it’s the origin of their magnetic fields. There are also suggestions that metallic hydrogen could be a superconductor, which makes it a very interesting material to study for possible applications here on Earth.’
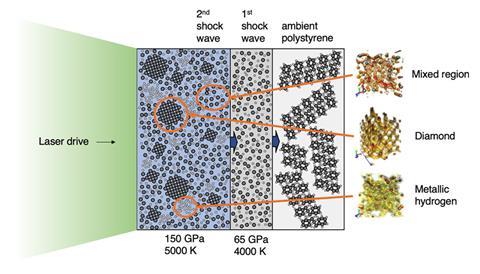
But compressing hydrogen to metallisation isn’t an easy task. ‘The main challenges are twofold: working with hydrogen and seeing what it’s doing,’ points out Hartley. ‘The first one is what we’ve tried to solve here – rather than using pure hydrogen gas, which is hard to contain or get to a high pressure, we’ve used plastic, which lets us start with a fairly high density of hydrogen atoms. At the same time, that makes the second problem harder. The main method we use to study what’s happening is x-ray diffraction. Unfortunately, atoms with more electrons give much more signal than atoms with fewer, so in our case, the carbon is 36 times easier to see than the hydrogen is.’
Hartley notes that even with the very bright x-rays they’re using, they can’t really see the hydrogen atoms. ‘Instead, we look for the effect that that hydrogen has on the behaviour of the carbon,’ he says. ‘From our simulations, we can tell that it isn’t mixed in with the carbon.’
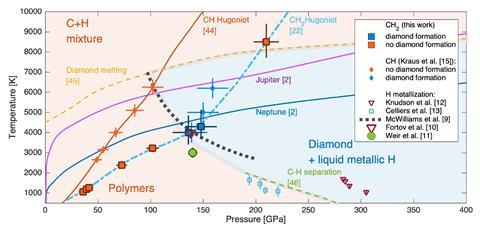
David Ceperley from the University of Illinois at Urbana-Champaign, US, who wasn’t involved in the study, notes that the team subjected ordinary plastics – polystyrene and polyethylene – to intense laser beams. ‘This resulted in shock compressing the samples up to approximately 1.5 million atmospheres pressure and 5000°C,’ he adds. ‘The researchers observed that the hydrogen and carbon that the plastic was made of separated into diamond and what they presume was liquid metallic hydrogen. They speculate that they may be able to measure properties of the hydrogen using a variety of new experimental techniques. If so, this is a new way of studying the interior of gas giants with hydrogen mixed with other elements.’
Hartley explains that when the plastic target is hit by the laser, the surface gets so hot that it explodes, putting huge pressure on the rest of the material. ‘But it can only stay at such high pressure for a very short time – around one nanosecond – so we use an even quicker x-ray pulse to see what happens before it explodes.’ Combining these experiments with ab initio simulations provides indirect evidence for the formation of metallic hydrogen, says Hartley.
But not everyone is convinced, however. ‘Combining experimental indirect evidences with theory is not a great way of obtaining new information,’ says Alexander Goncharov at the Carnegie Institution for Science in the US. ‘Theory may be simplified and the experiment simply does not record hydrogen.’
Dominik Kraus at the University of Rostock in Germany, who also took part in the research, points out that besides describing how to obtain liquid metallic hydrogen from simple plastics, he and his colleagues have outlined ways to use their observations to precisely characterise metallic hydrogen in the future. ‘For example, by adding high-resolution inelastic x-ray scattering methods as now available at x-ray free electron lasers,’ he says. ‘Such measurements are just not possible with the current highly elaborate sample environments involving pure hydrogen.’
Hartley agrees: ‘It’s right that we haven’t measured any new properties of metallic hydrogen in this study, but we don’t believe anyone before us had suggested that plastic would be an appropriate starting point to even try and measure such things.’
Kraus adds that there has already been intense discussion as to whether this indirect evidence is enough to prove the presence of metallic hydrogen. ‘The simple answer is that for now, there is no other known scenario that is compatible with our experimental observations. In science, you will never achieve absolute certainty on the validity of a model. It’s only possible to fully reject theories if they are incompatible with experiments.’
References
1 D Kraus et al, Phys. Rev. Res., 2023, DOI: 10.1103/PhysRevResearch.5.L022023
2 D Kraus et al, Nature. Astronomy, 2017, DOI: 10.1038/s41550-017-0219-9











No comments yet