Borrowed hydrogen chemistry drives reaction to obtain useful fuel from biomass
Scientists in the US have come up with a simple way to convert ethanol into 1-butanol, in what could be an important step forward for renewable energy.
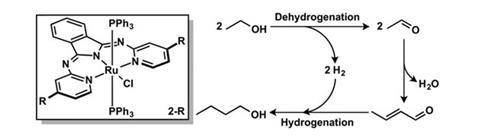
Ethanol can be made by fermenting biomass. However, ethanol presents problems as a fuel, such as low energy density compared to petrol, corrosiveness towards engine technology and fuel pipelines, and since it reacts with water, it can separate out from fuel blends over time.
Higher order alcohols like 1-butanol act more like petrol and can overcome many of these problems. Unfortunately, 1-butanol is hard to synthesise on a large scale.
Now, Nathaniel Szymczak and colleagues at the University of Michigan have used an amide-derived ruthenium complex as a catalyst for synthesising 1-butanol from ethanol with high selectivity and conversion under ambient conditions. The reaction dehydrogenates ethanol to the respective aldehyde, acetaldehyde. Two acetaldehyde molecules then react to form the conjugated aldehyde crotonaldehyde, which is then hydrogenated again to give 1-butanol. The role of the catalyst is to accept the hydrogen in the first step, and give it back in the third step, earning this type of reaction the description ‘borrowed hydrogen chemistry’.
References
This article is available for free until 25 February 2016
K T Tseng et al, Chem. Commun., 2016, DOI: 10.1039/c5cc09913g












No comments yet