The second ever praseodymium compound with a +5 oxidation state paves the way for pentavalent lanthanide chemistry
The first pentavalent praseodymium nitride–oxide that features a rare Pr≡N triple bond is the second ever lanthanide(V) complex to be made.1
Lanthanide chemistry is dominated by the +3 oxidation state. There are some common +4 lanthanide complexes such as cerium oxide (CeO2), but +5 compounds have proven elusive. Praseodymium has long been considered as the most promising route to lanthanide(V) chemistry as it has five valence electrons, but the first pentavalent praseodymium complex, the oxide PrO2+, was only synthesised last year.2
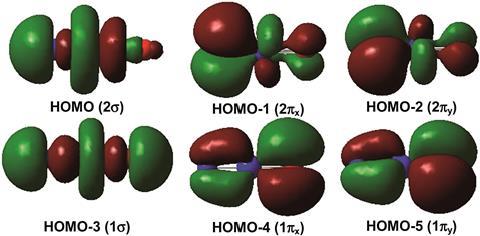
Now, a team led by Mingfei Zhou from Fudan University and Jun Li Tsinghua University, China, reacted individual praseodymium atoms with nitric oxide in a solid neon matrix at 4K (–269.15°C) to create the second ever lanthanide(V) compound, NPrO. The compound was detected using infrared spectroscopy.
Calculations show that its linear structure optimises the overlap of the praseodymium atom’s 5d and 4f orbitals with the nitrogen and oxygen orbitals. The 5d orbitals stabilise praseodymium’s high oxidation state – just as in PrO2+.
The participation of the contracted lanthanide 4f orbitals in covalent bonds is extremely rare and this research shows that higher oxidation state lanthanide chemistry could be richer than previously believed.
References
1 S-X Hu et al, Chem. Sci., 2017, DOI: 10.1039/c7sc00710h (This article is open access)
2 Q Zhang et al, Angew. Chem. Int. Ed., 2016, 55, 6896 (DOI: 10.1002/anie.201602196 )











No comments yet