Organo-lead compound completes +2 hydride puzzle of heavy group 14 elements
The first lead hydride with the oxidation state +2 has been made by German researchers, filling a gap in the low-valent hydrides of group 14 elements.
+4 hydrides such as silane (SiH4) and plumbane (PbH4) have long been a staple of inorganic chemistry. Although the corresponding bivalent hydrides, such as SiH2, are more unstable, most have been studied trapped in a matrix or coordinated to organic ligands. However, a +2 hydride of lead has been markedly absent from the line-up.
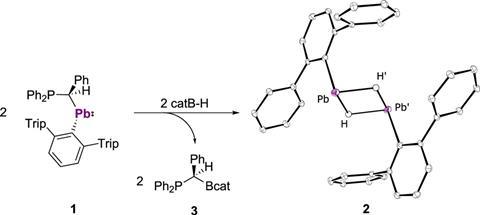
A team led by Lars Wesemann from the University of Tübingen has now isolated the first lead(II) hydride. They speculated the compound might be an intermediate of the hydroboration of plumbylene (a lead with one aryl and one alkyl substituent) into diplumbylene (lead bound to two aryl groups) and decided to slow the reaction down by cooling it to –20°C. This allowed the researchers to isolate the elusive lead(II) hydride, which comes as a dimer with two lead and two hydrogen atoms at its centre.
Wesemann’s team examined the structure using x-ray diffraction and proton nuclear magnetic resonance spectroscopy. The NMR hydride signals are the lowest field for any diamagnetic compound ever recorded.
References
J Schneider et al, J. Am. Chem. Soc., 2017, 139, 6542 (DOI: 10.1021/jacs.7b01856)














1 Reader's comment