Clever trick enables the design of a new class of material with interesting properties
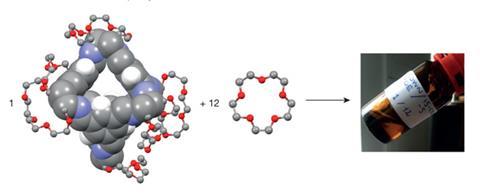
The combination of a rigid organic cage molecule and a bulky solvent has allowed researchers to produce liquids with permanent porosity.1 This new class of porous material combines the benefits of a liquid with those of a solid adsorbent framework and may find applications in important areas such as catalysis or carbon capture.
Porous solids, such as zeolites or metal–organic frameworks (MOFs), are commonly used in various chemical processes. These materials are structurally rigid and contain permanent cavities of regular sizes and shapes. By contrast, the ‘porosity’ in conventional liquids is limited to poorly defined and transient cavities between their molecules. Despite their many uses, the solid nature of zeolites and MOFs can impose limitations in several areas, so materials that combine both fluidity and permanent porosity are of great technological interest.
An international team involving scientists based in the UK, France, Germany and Argentina has now been able to design and prepare ‘permanently porous liquids’ using rigid organic cage molecules. These cages have the advantage that they can be dissolved in another material without breaking any of their chemical bonds, thus serving as soluble porous units. The authors point out that their porous molecular liquid differs fundamentally from porous macromolecular liquids containing hollow colloidal silica spheres.2 ‘We took these cage-like molecules, which have a hollow space inside that can be accessed through small windows, and dissolved them at very high concentration in a solvent whose molecules are too large to fit through the windows,’ explains lead author Stuart James, chair of inorganic chemistry at Queen’s University Belfast, UK. ‘In that way we created a liquid which has empty pores floating around in it.’
The authors first dissolved the cage molecules in a liquid composed of crown ethers – cyclic chemical units containing several ether subgroups. To achieve high solubility in the bulky solvent, the team functionalised each cage with six crown ether groups. Through this approach, they were able to obtain a concentrated liquid phase that flows at room temperature despite the high concentration of cages.
Trapped gas
‘The work by James and colleagues is important because it shows for the first time that a liquid can be made permanently porous,’ says Michael Mastalerz, professor of organic chemistry at Heidelberg University, Ruperto Carola in Germany. He explains that the challenge in creating such a liquid was designing a molecule that is either liquid by itself or highly soluble in a solvent that cannot penetrate the molecular pores. ‘This is not trivial,’ he says. ‘Usually, long or branched alky chains are employed to increase the solubility or lower the melting point of a larger molecule, but linear alkyl chains can penetrate into the cavities of adjacent molecules, resulting in non-porous liquids. James et al used a clever trick to solve this problem: they combined a porous crown-ether-decorated molecule with a crown ether.’
The researchers characterised the new material by combining molecular dynamics simulations with positron annihilation lifetime spectroscopy – a technique commonly used to study voids and defects in solids – and found that the porous liquids could dissolve unusually large amounts of gas. ‘Small gas molecules such as methane were able to get through the windows into the pores, so what we saw was that the pores massively increased the solubility of this gas,’ James says. The team demonstrated that cages with small peripheral groups could also be used to create porous liquids. This was achieved by making ‘scrambled’ cages from a mixture of commercially available reagents. In both cases, the secret was to avoid functional groups that could penetrate into the molecular cage cavities.
But according to Mastalerz, the surface area and the overall uptake of gases in the porous liquids are still much lower than those in most zeolites and MOFs. ‘At the moment this material cannot compete with those solids,’ he says, adding that permanently porous liquids should instead be seen as a prototype of a new class of substance. ‘Nevertheless, this material bears many advantages because it is a fluid and can for example be pumped through tubing and pipes. This may someday find technological applications in gas separation or transport.’
References
1 N Giri et al, Nature, 2015, DOI: 10.1038/nature16072
2 J Zhang et al, Angew. Chem., Int. Ed., 2014, 54, 932 (DOI: 10.1002/anie.201409420)









No comments yet