Scientists observe the development of mesoporous structures in real-time
A team of chemists from Spain and the US has for the first time watched the formation of large pores – mesopores – in zeolites.1 The researchers were able to follow the process in real-time thanks to a combination of state of the art experimental and computational techniques. They also demonstrated how some surfactants can act as scaffolds in the formation of mesoporous zeolites.
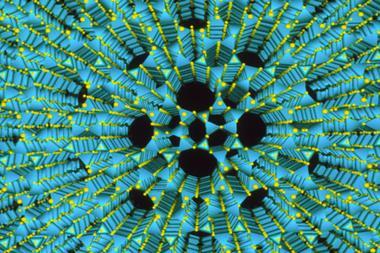
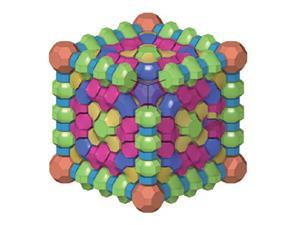
Zeolites are microporous crystalline materials with vast surface areas. Although these materials have myriad applications in catalysis, their use in industry is limited because bulky molecules can’t fit through the microscopic pores. To solve this, chemists developed mesoporous zeolites, which are now in high demand for heterogeneous catalysis.

A common technique to prepare mesoporous zeolites relies in the use of surfactants as templates for crystallisation. This technique was developed decades ago, inspired by the production of other mesoporous materials such as MCM-41.2 With bigger pores, large molecules can access the active sites more easily, making them useful for industrial applications such as oil refining. Some surfactant-templated zeolites show such remarkable activity and selectivity in oil refining that their production has passed the thousand tonne mark.3
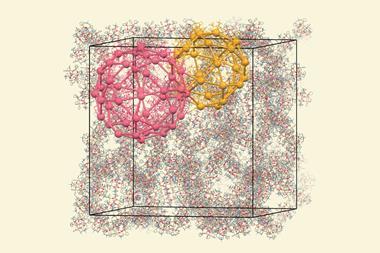
Understanding how surfactants act as templates for crystallisation could help scientists develop tailored catalysts for specific needs. Researchers, led by Javier García-Martínez, a nanomaterials researcher at the University of Alicante and co-founder of Rive Technology, combined in situ synchrotron x-ray diffraction and liquid-cell transmission electron microscopy (Liq-TEM) to monitor the formation of mesoporosity in real time. ‘Liq-TEM is a new technique that had never been used to study zeolites. It uniquely allows us to directly observe solids in a liquid solution with a very high resolution,’ says García-Martínez. ‘This has been an ambitious and demanding research project that required the collaboration of four international groups and over three years of experiments.’
David Sholl, professor at the school of chemical and biomolecular engineering at the Georgia Institute of Technology in the US, believes ‘the paper directs insight into controlling porosity in a material with considerable industrial interest […] bringing together a wide range of experimental and modelling techniques. The combination of scattering and real space microscopy is particularly valuable.’
García-Martínez dreams about the future of mesoporous zeolites. ‘We can already tune the size of the mesopores with tremendous accuracy (about 0.2nm) in a wide variety of zeolites,’ he says. ‘We would like to extend our surfactant-templating methods to other materials such as metal–organic frameworks and silicoaluminophosphates.’ Both García-Martínez and Sholl agree that tailored mesoporous zeolites could find new uses beyond oil refining. ‘Emerging applications such as biofuel processing will also be a fertile area for this kind of material,’ Sholl notes.
References
1 N Linares et al, Chem. Mater., 2016, DOI: 10.1021/acs.chemmater.6b03688
2 C T Kresge et al, Nature, 1992, 359, 710 (DOI: 10.1038/359710a0)
3 K Li, J Valla and J García-Martínez, ChemCatChem, 2014, 6, 46 (DOI: 10.1002/cctc.201300345)













No comments yet