Devices made with new electrolyte continue to operate under extreme conditions
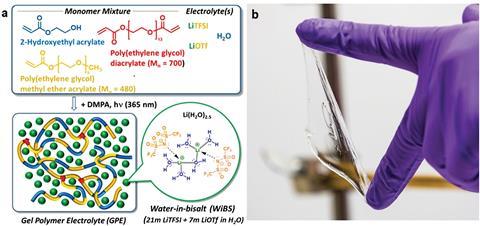
Researchers in the US have developed a lithium-ion battery electrolyte that doesn’t catch fire by combining an aqueous electrolyte with a gel polymer, thereby circumventing the major safety concerns of common flammable electrolytes.
‘The average person may possess multiple lithium-ion batteries in devices they use every day. Lithium-ion batteries’ high energy and rechargeability has revolutionised the way we use electronics – imagine if you had to recharge your phone every 10 hours, or worse, replace the battery every few days,’ says Konstantinos Gerasopoulos, who led the team at Johns Hopkins University Applied Physics Laboratory in developing the new electrolyte. ‘But what enables this high energy is exactly what makes them hazardous. If we can eliminate the safety hazards associated with lithium-ion batteries, we can transform the way that they are used in electronic devices.’
Compared to flammable organic solvents commonly used in battery electrolytes, water is inherently safe, but the use of aqueous alternatives is not always possible due to the instability of water against typical battery electrodes. However, water-in-salt and other concentrated aqueous electrolytes have attracted significant attention in recent years due to their stability in ambient environments.
Gerasopoulos’ group has taken this one step further by embedding an aqueous electrolyte containing a high concentration of two lithium salts dissolved in water (a water-in-bisalt electrolyte) into a polymer matrix to remove the liquid component altogether. This drastically reduces the chance of the electrolyte catching fire and expands its electrochemical stability, which is a significant step towards matching the voltage stability of organic electrolytes.
To test the performance and stability of their new electrolyte, Gerasopoulos subjected both the electrolyte and their flexible Li-ion battery system to a number of tests. ‘The key properties that we studied at the material level were the thermal and electrochemical stability, mechanical properties, and ionic conductivity. We ran over 100 full cell experiments to study capacity, cycle life and efficiency,’ he explains. ‘We even reported a test where we cycled the battery in ambient laboratory conditions, then burned it with a torch while powering a fan, and then resumed the test after cutting off the burnt portion. The battery continued to cycle for several days.’
‘Imagine having a battery like this in a smart wearable such as a garment that is subject to mechanical stresses,’ comments materials chemist Susan Odom from the University of Kentucky, US, ‘Having this polymer electrolyte as a flexible structural support could improve the durability of lithium-ion batteries for these purposes.’
Chibueze Amanchukwu, a molecular engineer who designs new electrolytes at the University of Chicago, US, hypothesises that water-in-salt electrolytes encapsulated in gel could be applied to electrocatalysis, where ‘extending the electrochemical stability window and limiting the hydrogen evolution reaction is paramount’. However, he has concerns about commercialising such electrolytes: ‘Salt is currently the most expensive component of an electrolyte. Water-in-salt systems need a large amount of salt – which equates to high cost.’
References
S A Langevin et al, Chem. Commun., 2019, 55, 13085 (DOI: 10.1039/c9cc06207f)











No comments yet