Lithium-ion batteries are an indispensable part of the transition away from fossil fuels thanks to their high voltage output and long cycle life, but their tendency to overheat and catch fire is a serious problem. Now two different papers have tackled different aspects of the problem: in the first, researchers in the US have imaged working battery electrodes with unprecedented resolution to ascertain why lithium batteries sometimes ignite when not on charge. In the second, researchers in China have developed an optical sensor that could be placed inside batteries to detect the onset of thermal runaway before it becomes dangerous.

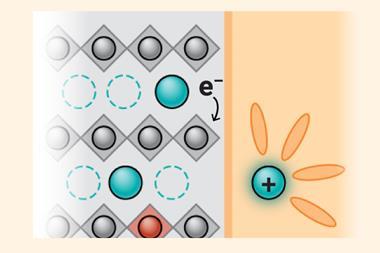
Intercalation electrodes were central to the development of functional, rechargeable lithium-ion batteries. ‘The gurus who came up with it gave the world a new kind of material … the fact that you can produce energy and release energy many thousands of times now is the miracle,’ says Nitash Balsara at the University of California, Berkeley. Simply plating the lithium onto an electrode causes significant capacity loss in every charge–discharge cycle. When the battery is charged rapidly, however, lithium may first plate onto the anode and subsequently intercalate into it owing to the flow of ionic currents that continue after the electric current has been switched off. ‘Between the lithium metal and the graphite you make a small battery,’ explains Balsara.
In the first paper, Balsara and colleagues used the Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory to perform micro x-ray computed tomography on the anodes of lithium-ion batteries after fast charging, measuring the density of the ionic current at different points. They found that the average ‘lithiation’ current was relatively modest. However, several hot spots had much higher currents, and these outliers persisted. The researchers suspect these unexpectedly high currents could explain reports of electric vehicles bursting into flames in garages. Balsara says that, at present, the group is still studying the fundamentals and destructive testing of batteries inside the ALS might prove impossible.
The second paper examines the more practical problem of characterising and detecting thermal runaway within working batteries. Most sensors cannot survive temperatures of up to 500°C that can arise during thermal runaway, which leaves engineers reliant on external detectors. This provide less detailed insight into the internal reactions. In the new work, researchers at the University of Science and Technology of China (USTC) and Jinan University, also in China, developed an optical fibre sensor that can be inserted into a working battery.
The researchers then etched into the sensor both a fibre Bragg grating and a Fabry–Perot interferometer. When illuminated with the same broadband light, the grating reflects a narrowband, temperature-dependent wavelength, whereas the interferometer reflects multiple resonances that depend on pressure. The same fibre, therefore, allows the researchers to track the pressure and temperature inside cells during thermal runaway.
They then placed the sensor in fully-charged commercial lithium iron phosphate cells and simulated overheating, finding that the cells initially heated rapidly with a relatively modest rise in pressure. However, a ‘shoulder’ was then reached, after which the temperature flattened out and the pressure began to shoot up rapidly. The electrolyte was now fully vaporised and further reactions – such as chemical decomposition – were irreversible. The researchers showed that the optical fibres had minimal effects on the cell’s performance and they therefore suggest they could be used as an early warning system to prevent thermal runaway. The sensors were not damaged by the thermal runaway process either.
Anna Stefanopoulou at the University of Michigan is excited by Balsara’s paper. ‘I really like the work showing the internal and unmeasured currents!’ she says. ‘We made a similar hypothesis about the internal current among parallel cells. These measurements are very important.’
Regarding the sensor that can track overheating, she adds, ‘I applaud this research effort where new sensing will inform and improve thermal management and safety.’ She is skeptical, however, that the technology will prove useful in the field, arguing that the same pressure and temperature can be measured externally with sufficient accuracy and much more cheaply. ‘Although [the researchers] say the fibre is inexpensive and can be part of the standard manufacturing, the data acquisition might be another story,’ she says.
Qingsong Wang of USTC and Tuan Guo of Jinan agree that ‘it’s not cheap’ but argue that it is a new technology that could be optimised and scaled up using a chip-based interrogator.
References
1 AS Ho et al, ACS Nano, 2023, 17, 19180 (DOI: 10.1021/acsnano.3c05470)
2 W Mei et al, Nat. Commun., 2023, 14, 5251 (DOI: 10.1038/s41467-023-40995-3)















No comments yet