Using less than 200 nanograms of einsteinium – half of the world’s supply at that time – scientists have uncovered the synthetic element’s bonding and spectroscopic behaviour for the first time.
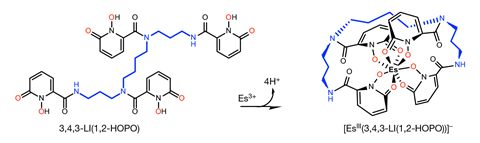
Discovered in the debris after the detonation of the first hydrogen bomb in 1952, einsteinium is a highly radioactive actinide. As it doesn’t occur on Earth naturally, little is known about its chemistry beyond the fact that it forms a few halide and oxide salts. Making more than just trace amounts of it means bombarding lighter elements with neutrons for a prolonged period of time – a process that can only be done at one place in the world, the high flux isotope reactor at Oak Ridge National Laboratory in Tennessee, US.
The lab’s latest efforts produced only 400ng of element 99, half of which went to a team led by Rebecca Abergel from the University of California, Berkeley, Corwin Booth from Lawrence Berkeley National Laboratory and Stosh Kozimor from Los Alamos National Laboratory. Despite working with less than 200ng of the element, the researchers managed to put einsteinium through x-ray absorption measurements, revealing its coordination chemistry and spectroscopic behaviour for the first time.
In some ways, einsteinium behaves similarly to its lighter neighbours on the periodic table, taking on a +3 oxidation state in a complex with an octadentate hydroxypyridinone ligand. The compound’s short einsteinium–oxygen bond length, however, came as a surprise. Luminescence spectroscopy measurements gave another unexpected result. ‘The way [einsteinium] changed upon complexation is in the opposite direction in terms of shifting wavelength and energy than what happens with the other actinides,’ explains Abergel. The team is now working on confirming why einsteinium’s behaviour is so different to other actinides.
Normal rules no longer apply
‘One thing that this speaks to is we don’t have a good handle on what the impacts of relativistic effects on the chemistry of these elements are,’ comments Jenifer Shafer, an expert in heavy actinide chemistry at the Colorado School of Mines, US. ‘The normal rules of quantum mechanics and electronic ordering – things like Hund’s rule – seem to dissolve as you get into this part of the periodic table.’
One of the most exciting things about doing actinide chemistry, is that they are elements that we get to write the textbook for
Stosh Kozimor, Los Alamos National Laboratory
Shafer is impressed with the Berkeley team’s ability to make the experiments happen in the first place. ‘I do a little bit of work with einsteinium, but I can’t imagine the challenge of trying to coordinate getting a few hundred nanograms and getting significant chemical data with it,’ she says.
‘You can’t actually see [the material when it arrives],’ recalls UC Berkeley researcher Katherine Shield, who did a lot of the bench chemistry. ‘It comes in a little vial, but the only way that you know that you’re actually working with it is by using radiation detectors.’ Apart from the miniscule amount the researchers had to work with, einsteinium’s 275-day half-life meant they were losing material as time went on. ‘You really, really hope that you don’t accidentally drop the one droplet while you’re doing the chemistry,’ Shield says.
Before even receiving the einsteinium, the team had carefully laid out the experiments they wanted to run and in what order, to minimise material loss. ‘We planned a lot in advance and nothing went according to that plan,’ laughs Abergel. Discovering that their sample contained high amounts of californium, they had to abandon planned x-ray diffraction experiments and turn to x-ray absorption spectroscopy, which allowed them to ignore the contaminant.
After preparing the einsteinium complex at Berkeley, team member Korey Carter then drove the precious – and hazardous – sample for an hour to the Stanford Synchrotron Radiation Lightsource. Once there, handling half of the world’s supply of einsteinium was ‘absolutely terrifying’, recalls Kozimor. ‘It takes a well-rehearsed team and steady nerves.’
‘It’s also a real privilege to be doing this kind of science,’ says Abergel. Understanding einsteinium’s chemical behaviour could help scientists produce and purify it with a view to making even heavier elements. ‘Einsteinium targets would make it more plausible to potentially identify the island of stability, which is a huge deal for nuclear physicists,’ explains Shield. The island of stability is a predicted set of superheavy isotopes that may have considerably longer half-lives than their near-neighbours.
For now, the team will keep working with the remaining einsteinium sample, carrying out electron microscopy, radioanalysis and separation experiments. ‘One of the most exciting things about doing actinide chemistry, is that they are elements that we get to write the textbook for. It’s truly exploratory,’ says Kozimor.
References
K P Carter et al, Nature, 2021, DOI: 10.1038/s41586-020-03179-3















No comments yet