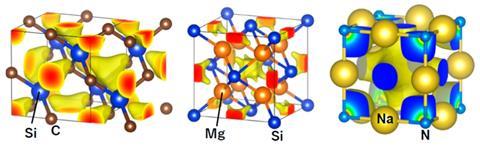
The unusual metallic behaviour of sodium nitride (Na3N) is caused by the empty spaces in its structure, researchers have discovered.
Sodium nitride was predicted to be an insulator as it has similar structural features to some other clathrates, for example sodium oxide chloride (Na3OCl). But unlike this white non-conducting material, Na3N is a blueish–black solid with electronic properties that resemble a metal.
The reason for this odd behaviour is that it has electronically active cavities, a team of chemists in Japan and Germany has discovered. They made the highly unstable compound by subjecting sodium or a liquid sodium–potassium alloy to nitrogen plasma. The cuboctahedral and square holes in sodium nitride’s three-dimensional structure are populated with conducting electrons from sodium’s s orbitals, with large electron density centred around the nitrogen atoms.
When nitrogen is introduced into sodium, two things happen: sodium forms ions and cavities open up in the structures as the sodium ions rearrange themselves to accommodate nitrogen. As the sodium ions move closer together, they form a conducting band of s electrons. Covalent interaction between this band and the cavities then collapses the band gap that would usually be present in a semiconductors or insulators, creating a metallic compound instead.
References
H Mizoguchi et al, J. Am. Chem. Soc., 2021, 143, 69 (DOI: 10.1021/jacs.0c11047)














No comments yet