Hundreds of helium compounds could be hiding in Earth’s mantle
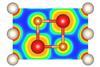
Although it doesn’t form any bonds, the noble gas can form compounds with salts at high pressure
Overturning conventional wisdom about the periodic table’s most unreactive element, US chemists have found that helium can form compounds with many salts – at the right pressures. This means there could be many more helium compounds than previously thought stored in Earth’s lower mantle, 700km below the surface.
In a surprise 2017 discovery, Artem Oganov from the Skolkovo Institute of Science and Technology in Russia and his colleagues found that helium forms a stable compound with sodium. ‘But even we thought at the time that [our compound was] an exception,’ admits Oganov, who wasn’t involved in the new study.
