Alkyne and oxazole groups allow each monomer to form four hydrogen bonds with its neighbours
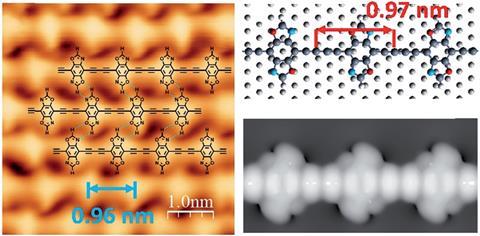
Harnessing the directing power of hydrogen bonds may be the answer to producing ordered and high quality 1D graphdiyne chains.
In recent years, the level of interest in 1D and 2D materials has increased dramatically due to their myriad properties giving rise to applications such as catalysis, sensors and electronic devices. While graphene is still the darling of this field, it is not the only player worth talking about.
Introducing graphdiyne – a close relative of graphene – the major difference is that, where graphene is a vast network of fused benzene rings, graphdiyne is based on a monomer consisting of a benzene ring with two acetylene groups on opposite ends.
With a lot of scope for tuning the direction of its growth and the size of its bandgap, graphdiyne is a versatile material. However, previous attempts to assemble it on a larger scale were hampered by unwanted side reactions and poor final results.
Now, a study led by Chenliang Su and Kian Ping Loh of Shenzhen University, China, has addressed this problem by adding oxazole moieties onto the monomer. This introduces the potential for hydrogen bonding.
When mounted on a silver surface, these monomers are able to form hydrogen bonds allowing them to self-assemble and adopt a close packed formation. Annealing this material forms covalent bonds between the units, resulting in highly ordered covalent networks with large surface areas and other desirable properties.
![An image showing the precursor 4,8-diethynylbenzo[1,2-d-4,5-d′]bisoxazole](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/web/w/z/t/jenstructure_639726.svgz)











No comments yet