Increase in aromaticity drives metallaaromatic ring contraction
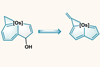
Acid-promoted ring contraction reaction reshapes an osmaindenol into an osmapentalene
Researchers in China have shown that one metallaaromatic framework can transform into another via a ring contraction reaction. Haiping Xia of Xiamen University, who led the work with Ming Luo of Southern University of Science and Technology, says this is the first aromatic framework rearrangement to be reported in an organometallic system.
