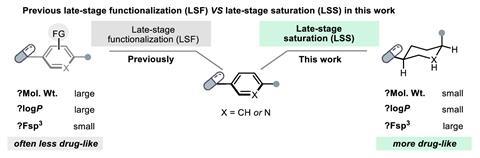
A general and quick strategy for converting complex aromatic building blocks into saturated molecules with improved medicinal properties has been developed. The new approach – called late-stage saturation – opens up opportunities in drug discovery and drug optimisation.1
‘The production of new medicines depends on the synthetic availability of suitable molecular structures, which usually have to be built up in several reaction steps,’ explains Frank Glorius at the University of Münster in Germany who led the study together with Tim Cernak from the University of Michigan in the US and Jiajia Ma at Shanghai Jiao Tong University in China. ‘It is time-saving and attractive if advanced, drug-like building blocks can be diversified. We show that aromatic hydrogenation under mild conditions is suitable for this purpose.’
Cross-coupling reactions have made it easy for scientists to synthesise aromatic molecules by simply clicking together a wide range of commercially available building blocks, but these rings don’t always make for good drugs. ‘Drug molecules often contain aromatic rings, resulting in structures that are flat. However, this flatness isn’t always advantageous for drug effectiveness,’ comments Michael Kassiou from the University of Sydney in Australia, who wasn’t involved in the study. ‘Converting these flat aromatic rings into their saturated forms enhances their three-dimensional structure, which has the potential to increase drug effectiveness.’ Previous work has shown that a higher aromatic ring count is often related to unfavourable characteristics such as poor solubility, selectivity and metabolic stability.2,3
Glorius points out that some starting structures already have favourable properties, so in many cases only a slight change is desired. ‘The hydrogenation of aromatic compounds is an attractive method because essential properties such as molecular weight and partition coefficient aren’t changed significantly, but a higher degree of saturation often proves to be beneficial,’ he says.
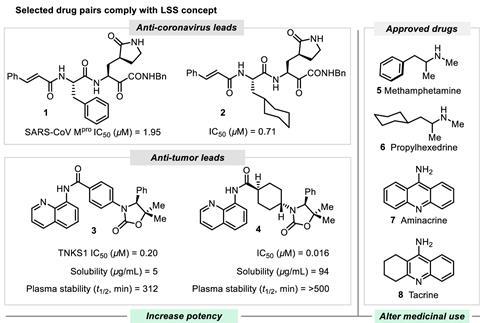
The team carried out a cheminformatic analysis and demonstrated that late-state saturation, where sp2 carbon atoms are converted into sp3 carbon atoms, can take place under mild conditions using a rhodium-based catalyst and a boron-based hydrogen source in an ethylene glycol solvent. After identifying the most suitable reaction conditions and protocols, they screened 768 pharmaceutically relevant complex molecules.
Glorius says that the rhodium-catalysed method is so powerful that even isolated benzene rings can be hydrogenated under mild conditions. ‘However, if the substrate contains other, more easily reducible groups – like a pyridine ring – these are usually preferentially hydrogenated,’ he adds. The approach tolerates trace amounts of dimethyl sulfoxide (DMSO) and water, which is important considering that pharmaceutical compounds are typically stored in DMSO.
The team demonstrated that late-stage saturation can also be achieved under acid-mediated reduction and photocatalysed-hydrogenation conditions. ‘These two protocols describe more specialised cases, such as the partial hydrogenation of indoles, and are intended to show that our strategy also works with other catalyst systems and conditions,’ notes Glorius.
David Sarlah at the University of Illinois in the US believes that the new approach could help to streamline drug optimisation. ‘While chemists have been developing similar approaches for decades, this work is by far the most comprehensive regarding the substrate scope,’ he says. ‘It also provides improved practical solutions focused on direct translation to the current drug discovery workflows. Therefore, I can imagine that many drug discovery programmes will quickly adopt it.’
References
1 D-H Liu et al, J. Am. Chem. Soc., 2024, 146, 11866 (DOI: 10.1021/jacs.4c00807)
2 F Lovering et al, J. Med. Chem. 2009, 52, 6752 (DOI: 10.1021/jm901241e)
3 TJ Ritchie et al, Drug Discovery Today, 2011, 16, 154 (DOI: 10.1016/j.drudis.2010.11.014)











No comments yet