Just three years after super-resolution fluorescence microscopy won the chemistry Nobel prize, another microscopy technique – cryo-electron microscopy (cryo-EM) – has just been propelled into the limelight to win this year’s chemistry prize.
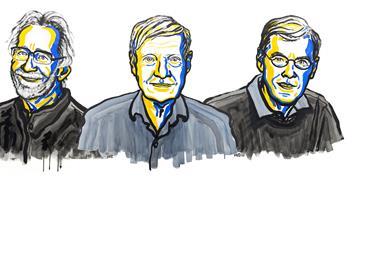
‘I was overwhelmed. I thought the chances of the Nobel prize were miniscule,’ Joachim Frank said in a phone call to the Nobel committee just after the announcement that named him, Jacques Dubochet and Richard Henderson as this year’s laureates. His feelings of surprise mirror those of the chemistry community as the laureate’s names didn’t appear in any predictions.
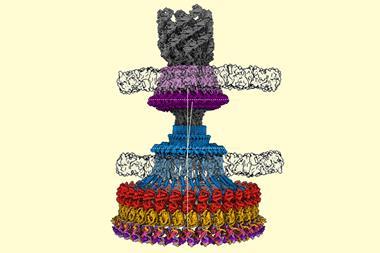
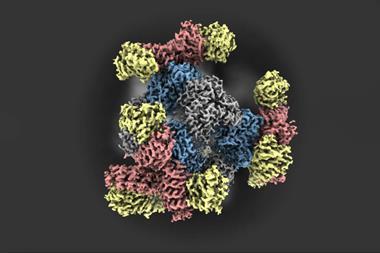
Dubochet, Frank and Henderson developed a method that made it possible to see the molecules of life for the first time as three-dimensional structures at atomic resolution. ‘This technique, long in the developing, has revolutionised structural biology allowing us to see the innermost workings of the tiny protein machines that run all of our cells,’ says Rob Kay, Henderson’s colleague at the MRC Laboratory of Molecular Biology, Cambridge, UK.
In many ways, today's #chemnobel is a testament to string-and-sealing-wax science: detectors, electronics, code, low-temp technology.
— Ashutosh Jogalekar (@curiouswavefn) October 4, 2017
Henderson was frustrated that x-ray crystallography, which requires samples to stay in rigid formations, wasn’t representative of biomolecules’ dynamic structures. So, he turned to electron microscopy, a method that had then been unable to resolve atomic details.
He kept proteins he wanted to study in their natural environment, a cell membrane, and covered the sample in a glucose solution. Thus protected from the microscope’s harsh electron beam, which quickly burns any living matter, he managed to obtain the first images of bacteriorhodopsin in 1975.
Want to learn more about cryo-EM? Read our explainer
In 1978, Dubochet from the University of Lausanne, Switzerland, was the first to discover that freezing samples rapidly in water protects them from the electron beam while avoiding the formation of ice crystals, which disturb the imaging. At the same time, Frank from Columbia University, US, found that he could reconstruct high resolution 3D images from multiple 2D electron microscopy traces.
‘A visual image is the essential component to understanding, often the first one to open our eyes, and so our minds, to a scientific breakthrough,’ says stem cell scientist Magdalena Zernicka-Goetz, from the University of Cambridge, UK. Protein complexes like the one that regulates the body’s circadian rhythm or enzymes involved in diseases like Alzheimer’s are now routinely imaged by cryo-EM. Recently, the Zika virus’ structure was resolved within a month.
By combining multiple cryo-EM images, scientists can now even make molecular movies that show biomolecules in action. ‘Medicine is no longer looking at organs, it is looking at processes in the cells,’ explains Frank.
‘One of the reasons that chemistry has always been regarded as so difficult and abstract is the fact that one cannot actually see the atoms and molecules directly,’ says chemist Andrea Sella from University College London, UK. ‘Cryo-TEM is also having huge impacts in materials chemistry,’ he adds. Polymer scientist Moatsou Dafni from University of Fribourg, Switzerland, agrees, mentioning on Twitter that cryo-EM’s abilities go beyond imaging biological structures.
I’m glad that the #ChemNobel went to a technique that is increasingly useful to (polymer) chemists.
— Dafni (@just_Dafni) October 4, 2017
Although scientists are clearly excited about this year’s prize, some bemoan the fact that one of the long-touted Nobel hopefuls, lithium–ion battery inventor John Goodenough, has been overlooked once more. Now 95-years-old, it seems unlikely that Goodenough will ever receive the nod from the Royal Swedish Academy of Sciences. ‘In terms of benefit to mankind, the lithium battery has gone hand in hand with our technological shift to portable, wearable tech,’ says Sella.
I fear the #chemnobel will never be #Goodenough
— Stuart Cantrill (@stuartcantrill) October 4, 2017
More information on cryo-EM and the Nobel laureates can be found on the Nobel prize website.













No comments yet