More than a quarter of the world’s population lacks access to safe drinking water. The task of water purification is further complicated by the need to keep energy use to a minimum, not only to reduce costs but also to avoid exacerbating climate change and further water scarcity. Water purification based on thermoresponsive hydrogels, are particularly attractive because they can soak up water like a sponge from a contaminated source and when wrung out release clean water using no other energy than sunshine. However, this process is slow. Now, Xiaohui Xu and colleagues led by Rodney Priestley at Princeton University in the US report ‘a breakthrough to overcome the inherent slow response rate’.
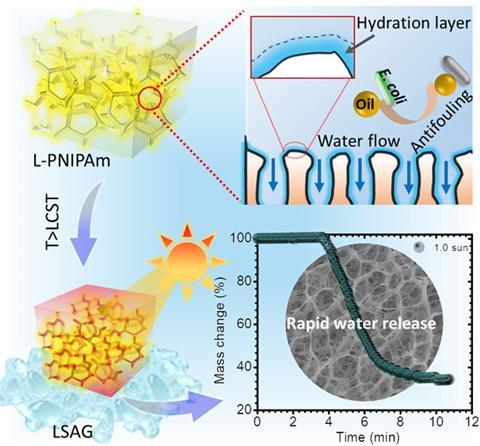
At a certain critical temperature, thermoresponsive hydrogels undergo a phase change, which changes their properties. The hydrogel Xu and colleagues studied – poly(N-isopropylacrylamide) (PNIPAm) – conveniently switches from being hydrophilic to hydrophobic at 34°C, so sunlight alone can flip it from soaking up water to releasing it cleansed of contaminants. However, as Xu points out, ‘The conventional PNIPAm hydrogel has a closed-pore structure, which forms a skin layer during water release and leads to a slow response rate.’ Taking inspiration from the loofah, which makes a great sponge, she and her colleagues have tackled these structural issues to significantly increase the water absorption and release rate.
The researchers modified the structure of the hydrogel to have open pores like a loofah by changing the polymerisation process. Xu tells Chemistry World how they stumbled on the approach while working on another project exploring the antifreeze properties of ethylene glycol (EG). ‘We found that when we polymerise PNIPAm in EG and water, the gel is opaque,’ she says, contrasting it with the transparency of the conventional closed pore hydrogel in water. Closer investigation with a scanning electron microscope revealed the open-pore loofah-like structure that had formed. The researchers put the structure change down to EG competing with the hydrogel for water and the poor solvency of PNIPAm in the water/EG mixture.

With this open-pore structure the volume of water absorbed increased by a factor of four, and when heated above the critical temperature the hydrogel released 70% of this water in just 5 minutes. The conventional closed-pore PNIPAm hydrogel releases only 3% of its water over the same timeframe.
Microplastics, which are too large to navigate the open pore network of the loofah-style PNIPAm hydrogel, are naturally filtered out. In addition, the researchers functionalised the hydrogel with polydopamine, which has a high affinity for contaminants like dyes and heavy metals, trapping them. They also functionalised it with zwitterionic poly(sulfobetaine methacrylate). The hydration layer that forms as a result prevents oil and microorganisms from adhering so that they are not adsorbed along with the water, giving it antifouling properties. The water collection rate of the functionalised open pore hydrogel was 26.88kg/m2/h, around four times that with the closed pore structure.
Next the researchers plan to test the gel’s ability to purify water contaminated with polyfluoroalkyl substances, which have been raising health concerns. They are also working on developing an antibacterial hydrogel that can efficiently kill waterborne bacteria.
References
X Xu et al, ACS Cent. Sci., 2023, DOI: 10.1021/acscentsci.2c01245
















No comments yet