Hydrogen atom bound directly to iron detected at ultra-low shift by paramagnetic nuclear magnetic resonance for the first time
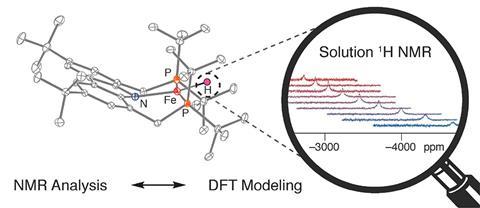
For the first time, a 3d metal-bound hydrogen atom was detected by paramagnetic solution nuclear magnetic resonance (NMR) spectroscopy. With the help of computational modelling, chemists have pinned it down at –3560ppm – the most extreme upfield proton chemical shift ever found.
Most chemists are used to the sharp peaks and refined spin–spin coupling patterns in diamagnetic proton (1 H) NMR. But spectra of paramagnetic compounds – those with unpaired electrons like radicals or some metals – look different: they have broad signals and a chemical shift range of around 200ppm.
For example, the hydrogen atoms in diamagnetic ferrocene have a chemical shift of around 4ppm, but paramagnetic dimethylnickelocene’s protons can be found at 200 and –200ppm. Hydrogen atoms bound directly to a paramagnetic metal have even more extreme shifts, and until now hydrides bound to 3d paramagnetic centres had never been detected by solution NMR.
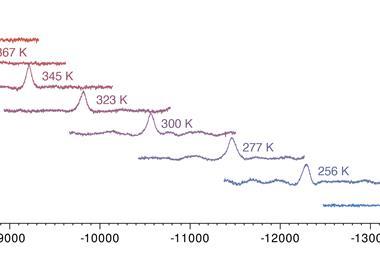
Chemists have now found a hydrogen atom bound to iron in a square-planar complex at –3560ppm – the most shifted proton signal recorded to date. The signal’s position was also extremely temperature dependent, shifting by 2000ppm between –50 and 110°C.
In order to target the right chemical shift range, the researchers had turned to density functional theory calculations. The simulations had predicted the H at –3770ppm.
Paramagnetic metal compounds like their planar iron complex can catalyse reductions, the researchers point out, which is why characterising them is important.
Note: this article was amended on 3 January 2019. The original erroneously reported that no hydrogen atoms bound to paramagnetic metal centres have ever been detected by NMR
References
J C Ott et al,J. Am. Chem. Soc., 2018, 140, 17413 (DOI: 10.1021/jacs.8b11330)
















No comments yet