An unusual mathematical model has recreated the intricate crystal growth patterns of chemical gardens, finally explaining how these beautiful, lifelike structures form.
These intriguing formations of inorganic crystals have fascinated scientists for hundreds of years and the chemical reactions which create these precipitates are well-understood. ‘When you drop a metal salt crystal into a solution of sodium silicate – also known as waterglass – it starts to dissolve. This forms a thin skin around the crystal, mostly made of insoluble metal hydroxides,’ explains Oliver Steinbock, a microstructures chemist at Florida State University, US. ‘Meanwhile, osmosis is at work, pushing water through this semi-permeable membrane. This building pressure eventually causes small breaches, which shoot out a jet of salt solution. Around these jets, the tubes of our chemical garden begin to take shape.’
Previous theoretical studies have attempted to explain the unique shapes created by this simple reaction but these striking and complicated crystal deposits have proven difficult to model accurately and the underlying physical mechanisms that determine the structures of chemical gardens remained a mystery. ‘The chemistry itself is simple – it’s the interaction of chemistry with physics that makes chemical gardens so complicated,’ explains Julyan Cartwright, a dynamical-systems physicist at the University of Granada in Spain. ‘There are three things involved: there’s the chemistry, there’s the solid mechanics of membrane formation and there’s the fluid flow from the accompanying osmosis. It becomes impossible to do rigorous fundamental mechanics on these systems.’
To simplify modelling a growing chemical garden, Steinbock’s team developed a higher level algorithmic model known as a cellular automaton, which uses known characteristics of a system to predict future outcomes. The team defined a series of rules dictating the growth of individual membranes within a chemical garden, applying these conditions over a series of intervals to investigate how the overall structure evolves. ‘In the 2D model, chemical gardens (C) form via the injection of a salt solution (B) into a thin waterglass layer (A), modelled on a grid where each point represents either A, B or C,’ explains Steinbock. ‘We assume that i) the proximity of A and B creates C and that ii) with each time step, a new B site is added by solution injection, moving a C outwards.’
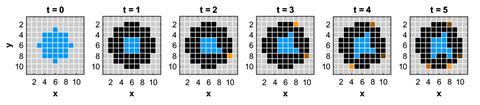
The team slowly increased the complexity of the model, tweaking the existing rules to simulate the system in three dimensions and account for additional physical parameters such as buoyancy and reaction rate. These analyses identified membrane ageing as the dominant process giving rise to the varied structures seen in experiments, with younger material creating more intricate filament-like shapes. ‘Fresh material appears elastic and can expand its area and mass irreversibly via microscopic breaches that bring the reactant solutions in contact to form additional material. The old material is more brittle and tends to form single, larger breaches,’ says Steinbock. ‘This finding is significant because it allows us to think about designing materials that can respond to environmental changes, self-heal, and even grow, much like living organisms.’
Cartwright is impressed by the scope of Steinbock’s model and is intrigued to see how this work will translate to other systems. ‘This type of model is capable of exploring a large parameter space. You can tune and change parameters and see how that changes the form of the product you’re making,’ says Cartwright. ‘I think it would be interesting to explore the limits of the parameters that conform with the model and how it can help us understand the origin of life or natural systems such as hydrothermal vents.’
Steinbock’s team are currently working on expanding the cellular automaton model to encompass additional parameters across more diverse systems and are using this knowledge to study the role of these materials in prebiotic chemistry – something that could shed light on the origin of life.
References
BC Batista, AZ Morris and O Steinbock, Proc. Natl. Acad. Sci. USA, 2023, DOI: 10.1073/pnas.2305172120















No comments yet