A high throughput, single molecule test based on nanopores and DNA barcoding has been developed and it could help uncover aggregates of misfolded proteins implicated in Alzheimer’s and Parkinson’s disease. The test could help improve diagnoses of these diseases, as well as tracking their progression.
While the characterisation of harmful oligomers is crucial to developing more accurate diagnoses and breakthrough treatments for diseases that involve misfolded proteins, identifying them in complex mixtures has remained a challenge.
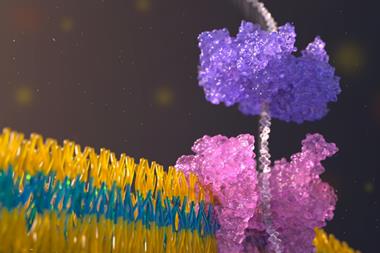
Nanopore sensors have been in the works for several decades and have great potential to detect a wide range of molecules rapidly. ‘[They] enable the detection and quantification of molecules by driving them through a nano-sized opening,’ explains Ulrich Keyser at the University of Cambridge and one of the study’s lead authors along with his colleague Michele Vendruscolo.
Broadly speaking, there are two types of nanopore: pore-like proteins embedded in membranes and solid-state ones, fabricated by creating nano-sized openings in a material.
‘One of the major successes of biological nanopore sensing is the possibility to perform DNA sequencing, as commercialised by Oxford Nanopore Technologies,’ explains Joshua Edel at Imperial College London, who was not involved in the project but has conducted similar research. ‘There is an unmet need to develop platforms that can rapidly detect a large array of biomarkers directly from patient samples. This offers the possibility for performing longitudinal disease tracking or even early disease detection.’
This is where solid-state nanopores have an advantage. They allow for direct measurements by eliminating the need to separate a sample, and pore size can be tuned to suit the target analyte – even those that have been difficult to quantify in complex biological samples. In this work the team created 10–15nm wide nanopores in a polymer membrane using commercially available quartz capillaries.
‘Oligomers are transient, conformationally heterogeneous, and present at very low concentrations,’ explains co-author Robert Horne. ‘There are thus very few methods sensitive enough for detecting them alongside cell components while effectively differentiating them from monomeric proteins and larger aggregates.’
In typical solid-state nanopore sensors, the nanoporous material is immersed in an electrolyte solution and electrodes are placed on either side of the pores, creating an electric field across them. Biomolecules of interest are introduced into the electrolyte and the electric field drives them through the pores one at a time. ‘As molecules move through, the liquid containing salt ions is displaced,’ says Keyser. ‘The drop in liquid volume correlates to an increase in resistance, and thus a drop in current.’
This change in current can be used to determine the weight, conformation and charge of molecules present in the sample. However, a challenge is the rapid speed at which molecules move through the pores, leading to variability in results and limited resolution.
‘To solve this, we used customisable DNA nanostructures and bound proteins to them,’ says Sarah Sandler, a PhD researcher in the Keyser group. ‘Using the current signal, we observe a “barcode” created using DNA nanostructures. Next to this barcode, we have a small piece of DNA tagged with a chemical group that can bind only to the protein oligomers, creating an additional spike.’
The advantage is every oligomer can be clearly identified and aggregates from different screens can be mixed and tested simultaneously, enabling investigation in greater detail and at higher throughput than previously possible.
As a proof-of-concept, the DNA nanostructures were designed to bind to oligomers of α-synuclein – the protein implicated in Parkinson’s. They also studied the rate of oligomer formation in the presence of several small molecule inhibitors of α-synuclein aggregation.
The team demonstrated comparable performance to micro free-flow electrophoresis, an existing single-molecule technique that allows full characterisation of oligomers under biological conditions. The advantage of the nanopore sensor is a greater potential for high throughput testing and scale up.
‘The ability to characterise individual protein complexes, particularly those undergoing dynamic assembly, has been of high interest,’ says Yujia Qing, an organic chemist at Oxford University, who was not involved in the study. ‘This not only offers promise for future inhibitor screening but also presents the potential … for early diagnosis of Parkinson’s disease.’
References
SE Sandler et al, J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.3c09335











No comments yet