Scientists in Czechia have devised a new method to assess the covalency of halogen bonds.1 By evidencing to what degree electrons are shared in this context, the method will contribute to ongoing debates on the physical nature of these increasingly important supramolecular interactions.
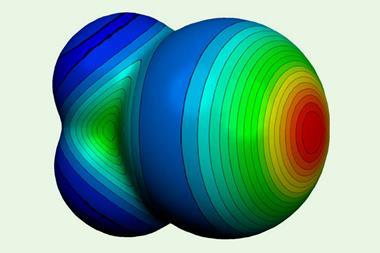
Akin to better-known hydrogen bonds, halogen bonds are crucial in the fields of catalyst, drug and materials design. They occur between different molecules or within the same molecule when a nucleophilic region, such as a lone pair of electrons, on one atom is attracted to the electrophilic region, the σ hole, on a halogen atom. And they depend on the polarisability and electronegativity of the halogen (X) as well as the electron withdrawing capacity of the group to which the halogen is bonded to (R).
Despite halogen bonds being understood to be a electrostatically-driven noncovalent interaction, ‘by now there’s lots of evidence that there’s a covalent contribution’, says Robin Perutz, an inorganic chemist at the University of York in the UK, who wasn’t involved in the research. Investigations into how covalent these interactions are typically involve interrogating crystal structures with techniques such as x-ray diffraction or infrared (IR) spectroscopy. These techniques show the change in R–X bond length caused by halogen bonding.2
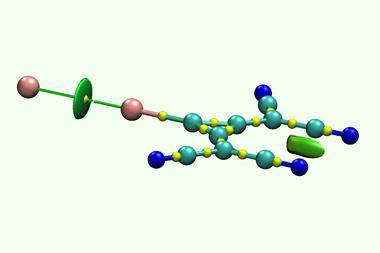
Now, Radek Marek at Masaryk University and colleagues propose using paramagnetic nuclear magnetic resonance (NMR) spectroscopy as an alternative technique to probe the covalent nature of halogen bonds. ‘This method provides high sensitivity and detailed information beyond common experimental techniques such as x-ray diffraction or IR spectroscopy,’ says Marek.
Marek’s team compared the 13 C NMR spectra of halogen bonded cocrystals with either paramagnetic or diamagnetic metal complexes. They found that the peak corresponding to the carbon bonded directly with the halogen (C1) shifted significantly between the paramagnetic and diamagnetic metal cocrystals. This hyperfine shift is caused by various interactions between the nuclear and electron spins, including the Fermi contact interaction.
‘Fermi contact contribution to the NMR shifts, related to the intermolecular electron spin transmission [of the metal] to the probe nucleus [C1] is an excellent indicator of electron sharing in … halogen bonded cocrystals,’ explains Marek.
‘Our study provides direct, highly sensitive experimental evidence for covalency in halogen bonds,’ says Marek. Although non-covalent interactions remain the dominant feature of halogen bonding, Marek’s team has shown that covalent interactions, ie electron sharing, can account for as much as 25% of the total interaction energy.
‘The method is very nice,’ says Perutz, but ‘you could take this a lot further’. Because hyperfine shifts vary with temperature, Perutz is surprised the team did not investigate the temperature dependence of paramagnetism to rule out other competing contributions. He recommends further NMR studies probing adjacently bonded fluorines, which could reveal even greater covalent detail.
Perutz also questions whether more sensitive techniques are already available, noting that ‘there are other situations where you can get much bigger shifts’.2,3
Still, both Marek and Perutz say that refining our understanding of halogen bonding is vital for increased accuracy in modelling the interactions of catalysts, functional materials and pharmaceuticals.
References
1. R Marek et al,Chem. Sci., 2025, 16, 20239 (DOI: 10.1039/d5sc05769h)
2. V Thangavadivale et al,Chem. Sci., 2018, 9, 3767 (DOI: 10.1039/c8sc00890f)
3. AC Castro et al,Inorg. Chem., 2023, 62, 4835 (DOI: 10.1021/acs.inorgchem.2c04063)













No comments yet