Almost 60 years after the first organo–plutonium complex was reported, researchers have prepared the first complex with a plutonium–carbon double bond. They say the finding provides the opportunity for chemists to understand more about the divergence in properties and chemical reactivity between actinides and lanthanides.
The first organo-plutonium complex (Pu(C5H5)3) was reported in 1965 but research into the fundamental properties of plutonium has been held back due to experimental difficulties and availability of the element. ‘Uranium is probably the last element in the periodic table where you can work in a normal laboratory,’ explains Steve Liddle, head of inorganic chemistry at the University of Manchester and one of the researchers on the study. ‘Go one place to the right and you need a completely different radiological lab setup.’
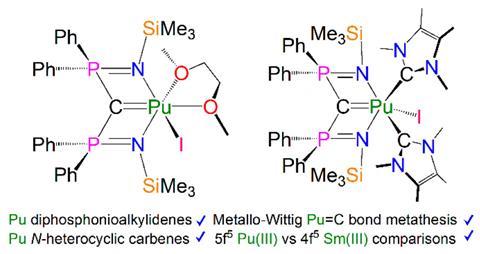
Liddle explains that due to the paucity of plutonium compounds there has been little opportunity to make experimental comparisons between the trans-uranium elements and the lanthanides but the act of making them, testing them and studying them has enabled them to do just that. ‘These are the first double bonds to carbon with a trans-neptunium system, ie plutonium,’ he says. ‘There’s also the first N-heterocyclic carbene–plutonium interactions.’
He says the work has provided an insight into the experimental differences between lanthanides and actinides, in terms of the Wittig chemistry they carried out to make the carbon–carbon double bonds but no such reactivity with lanthanides, and the distinct divergence in their electronic structure.
‘It is the generally held view that if you go left to right in the actinide series, the actinides become more lanthanide-like, but this study suggests that plutonium isn’t there at that point, you have to go further right. So this is helping people to get a handle on where you are on that scale. But we’re seeing real experimental divergence in properties and chemical reactivity between plutonium and analogous lanthanides.’
References
J Murillo et al, J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.3c12719
















No comments yet