Solvent decides bonding battle winner in supramolecular systems
Solvent effects control competition between hydrogen bonding and halogen bonding in supramolecular systems, new research shows. The upshot of the finding is a potential new tool to direct supramolecular self-assembly.
During self-assembly, each molecule breaks its bonding interactions with neighbouring solvent molecules, then forms new interactions. To investigate competition between hydrogen bonding and halogen bonding when co-crystals form, researchers from an ongoing collaboration between the UK Universities of Sheffield, York and Cambridge chose seven solvents of different polarities to study three aromatic molecules known to self-assemble. The molecules’ functional groups included pairs of hydrogen bond and halogen bond donors that compete for a common acceptor group.
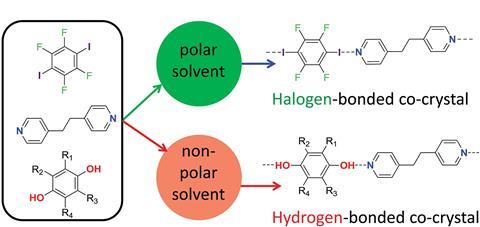
Lee Brammer, a senior contributor to the work from the University of Sheffield, explains the similarities between these two types of intermolecular interactions: ‘To form a hydrogen bond, a covalently-bound hydrogen atom carrying a partial positive charge interacts directionally with an electron-rich site – such as an electron lone-pair – on a neighbouring molecule. In a halogen bond, a covalently-bound halogen atom – usually iodine – interacts in a similar directional manner with an electron-rich site via a partially positively-charged polar region on the halogen atom.’
In most of the experiments, solvent choice directed the exclusive formation of either the hydrogen-bonded or the halogen-bonded co-crystals. More polar solvents weakened hydrogen bonding in solution and favoured softer halogen bonding, whereas less polar solvents favoured hydrogen-bonded co-crystals. Using toluene resulted in exclusively hydrogen-bonded co-crystals, while halogen bonding occurred within most of the co-crystals formed in isopropanol. ‘Solvent can play a significant role in directing the outcome of the synthesis and should be considered as part of the synthetic strategy,’ comments Brammer.
The group also identified borderline solvents that did not clearly favour either hydrogen or halogen bonding and found that the crossover point in solvent polarity correlates with the relative strength of the hydrogen and halogen bonds in the co-crystals. This is the first discovery of the prominent role of the solvent in selecting the preferred intermolecular interactions in co-crystal formation.
Jon Steed, a supramolecular chemist at Durham University, UK, says these findings could provide a useful ‘rule of thumb’ for synthesising co-crystals. By designing co-crystals with selectable intermolecular bonding interactions, scientists can control solid state properties such as solubility and dissolution rate. And Steve Scheiner, a computational chemist at Utah State University, US, envisions that altering solvent polarity could even switch between different types of hydrogen bond.







No comments yet