Rare halogen bond featuring phosphine
Scientists in Canada have engineered a very unusual cocrystal that contains halogen bonds between phosphine and organoiodine.
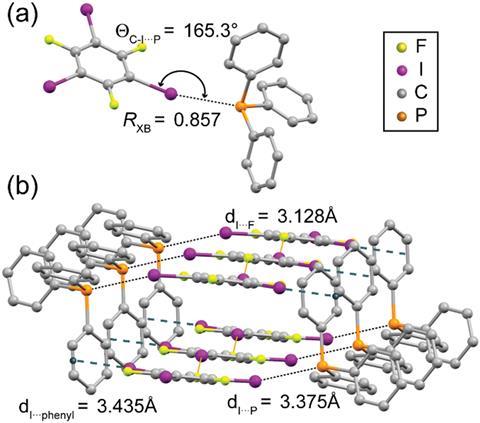
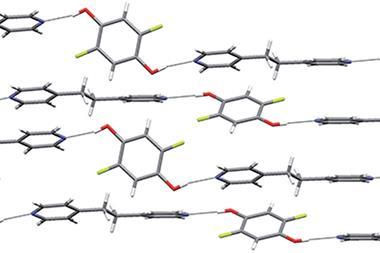
Halogen bonds feature in many areas of chemistry and are especially important in crystal engineering and organocatalysis. But halogen bonding involving phosphine is rare because the strongly polarising nature of phosphorus means it is more likely to form a covalent bond with a halogen atom. David Bryce and colleagues from the University of Ottawa, however, have prepared a cocrystal of triphenylphosphine and 1,3,5-trifluoro-2,4,6-triiodobenzene that has a strong and linear phosphorus–iodine halogen bond. Bryce’s team also showed that the phosphorus–iodine halogen bond is present in solution.
Noncovalent interactions are very important in deciding the properties of structures so the researchers’ hope their work could steer crystal engineering in new directions.
References
This article is free to access until 13 November 2018
Y Xu et al, Chem. Commun., 2018, DOI: 10.1039/c8cc06019c







No comments yet