Structures that mimic the diving bell spider’s air-trapping hairs boost the catalytic activity of copper electrodes
Inspired by the only spider that can live underwater, researchers have developed a hydrophobic copper catalyst that boosts the conversion of CO2 into fuels such as ethylene and ethanol.
Fossil fuels are our most important source of energy and chemical feedstocks, but are also major contributors to global CO2 emissions. The electrocatalytic reduction of CO2 could provide sustainable solution to this dilemma.
‘We were inspired by the diving bell spider, which traps a big air bubble near its abdomen using a dense layer of super-hydrophobic hairs,’ explains Victor Mougel from ETH Zurich in Switzerland, who led the study. ‘These structures had been previously used to create self-cleaning devices or anti-fog products. We decided to give them a try in electrocatalysis, and they work wonderfully.’
To mimic the spider hairs, Mougel’s team created copper electrodes covered in hierarchically structured dendrites. ‘We easily grow them through electrodeposition,’ he explains. ‘Then, we dip them into a waxy alkanethiol that binds to the copper, covering it with a monolayer that, despite being just two nanometers thick, transforms the electrode into a super-hydrophobic material.’
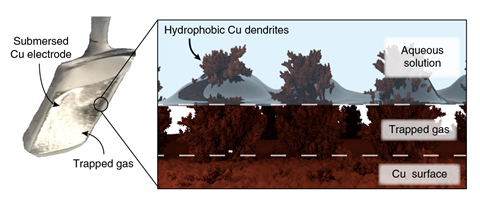
Ifan Stephens, an expert in electrocatalysis from Imperial College London, UK, calls the work ‘a very elegant proof of concept’. ‘Thanks to the hydrophobic copper structure, the researchers manage to trap air bubbles right at the catalyst surface, increasing the CO2 concentration near the active sites,’ he says. ‘It also provides a compelling explanation for earlier observations that the presence of hydrophobic elements, such as copper oxide or Teflon, can drastically improve CO2 reduction.’
Melanie MacGregor, an expert in wetting of nanostructures from the University of South Australia, says it is ‘exciting that someone is finally using the trapped nanobubbles effect to do something useful’.
‘The coating strategy is particularly neat, making copper perfectly hydrophobic without masking its topography, which would impede the catalytic reaction,’ she adds.
The new catalyst reduces the formation of hydrogen gas – an unwanted side reaction in aqueous CO2 reduction – and favours the production of multicarbon products. The team obtained high quantities of ethylene and ethanol, both valuable as fuels or building blocks for the chemical industry. ‘This is thanks to the hydrophobicity of the copper dendrites,’ says Stephens. ‘Without the trapped gas bubbles, you would be limited by the low concentrations of CO2 dissolved in water.’ Mougel explains that carbon monoxide could also be key: ‘Carbon monoxide is an important intermediate in the formation of multicarbon products that generally escapes water due to its low solubility, but in this case also remains trapped in the gas bubbles near the electrode.’
Stephens argues that the method needs improvements before it can be implemented in real devices. Mougel points out that the team is ‘aware of the downsides, and working to develop better solutions’. Both are hopeful that further studies into hydrophobic materials will pave the way for exciting new developments in electrocatalysis.
References
D Wakerley et al, Nat. Mater., 2019, DOI: 10.1038/s41563-019-0445-x











No comments yet