
The addition of a simple sugar, β-cyclodextrin, can boost the performance of flow batteries. A team from the US Department of Energy’s Pacific Northwest National Laboratory (PNNL), dissolved the starch derivative in the flow battery’s electrolyte, leading to a 60% improvement in the battery’s peak power, and boosted its capacity and longevity.
Flow batteries show promise for storing energy from intermittent sources such as wind and solar, later releasing it to the grid at times of high demand. The simplicity of their design means that scaling them up is straightforward, so they have potential for large scale energy storage. The batteries consist of two chambers that each contain a different electrolyte. Ions can cross a membrane between the two electrolytes, and this results in a flow of electric current to an external circuit.
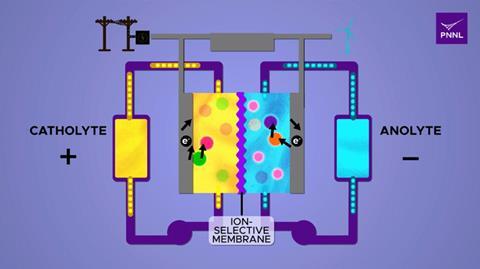
The team found that adding β-cyclodextrin to the aqueous electrolyte helped improve storage of positive charge and balanced out the flow of the electrons as the battery discharged. Dissolved in the solution as a homogeneous catalyst, the β-cyclodextrin helped to speed up the electrochemical reaction. The addition of the sugar also allowed more fluorenol – one of the molecules that holds charge in the battery – to be added. The group from PNNL experimented with the amount of β-cyclodextrin added to the electrolyte and discovered that adding too much led to the electrolyte becoming too thick limiting the rate of the flow, while too little and the performance of the battery suffered.
The team cycled the battery repeatedly for over a year, resulting in barely any loss of its capacity or ability to recharge. The test only ended when the battery’s plastic tubing began to fail.
‘We were looking for a simple way to dissolve more fluorenol in our water-based electrolyte,’ explains author Ruozo Feng. ‘The β-cyclodextrin helped do that, modestly, but its real benefit was this surprising catalytic ability.’
References
R Feng et al, Joule, 2023, DOI: 10.1016/j.joule.2023.06.013














No comments yet