A new class of molecules based on just main group elements can store and release energy efficiently in flow batteries, with minimal degradation. Although the work is purely a proof-of-concept, researchers in the field are excited by the possibilities for energy storage. ‘It’s great… extreme potentials and high stability,’ says Gloria De La Garza, a doctoral candidate working on redox flow batteries at the University of Michigan, US, who wasn’t involved in the study.
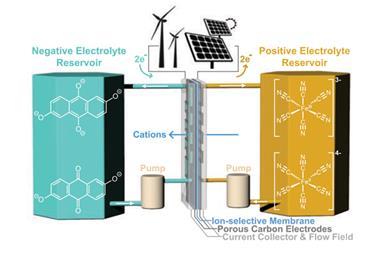
Redox flow batteries are an attractive way to store energy from intermittent sources, such as solar and wind because of their simplicity and the potential to scale them up easily. In a flow battery, electrochemical energy is stored on soluble molecules, housed in spatially separated storage tanks. ‘As the name suggests, flow batteries are dynamic; electrochemical energy is released from a redox event between the [different] species that is controlled in flow,’ explains lead author Máté Bezdek, from ETH Zürich in Switzerland. Flow presents a unique opportunity ‘to independently tune the amount of energy stored and the rate [of] release’, adds Bezdek.
However, most technologies today face limitations, including low voltages, but mostly the reliance on scarce materials and metals like vanadium and corrosive compounds such as concentrated sulfuric acid. ‘That’s why researchers explore redox-active organic molecules for flow batteries,’ De La Garza explains.
Now, researchers have discovered an organic molecule, made of main group elements – including phosphorus and sulfur – that can store charge efficiently. ‘Our new system expands the cell voltage achievable with organic systems with a surprisingly high degree of stability,’ says Bezdek.
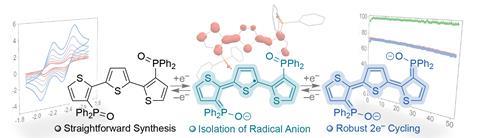
The team designed different terthiophenes – structures with three thiophene heterocycles linked together. ‘We chose [them] because, theoretically, they store electrons at extreme potentials,’ explains Bezdek. They then added phosphine oxide functionalities as strong electron acceptors, to stabilise the terthiophenes in charged states. It worked perfectly ‘without compromising high cell voltages’, says Bezdek. ‘We’re also excited … that this completely new electrolyte platform [is] synthesised in a straightforward manner,’ he adds. It’s just a couple of simple steps from commercially available reagents. This could ‘offer opportunities to tune performance with further structural modifications’, he adds.
‘This is the most negative anolyte [electrolyte on the anode side of the cell] reported, and it stores two electrons,’ says De La Garza, who had ‘never seen’ functionalisation of redox-active molecules with phosphine oxides until now. ‘This breakthrough could help stabilise other systems,’ she says. ‘I wonder how many other commonly irreversible molecules could become viable for redox flow batteries if functionalised with this [phosphine oxide] group.’
‘New chemistries bring valuable knowledge [for] electrochemical energy storage technologies,’ says Ana Jorge Sobrido, an expert in flow batteries at Queen Mary University of London, and a committee member of the UK Redox Flow Battery Network. Usually, organic systems convey compromises between battery capacity and durability. ‘This doesn’t seem critical in terthiophenes, [which] exhibit high storage capacity retention and multielectron storage at significantly negative voltages,’ she says.
‘The longer we reliably charge and discharge without degradation, the better,’ explains Bezdek. Tests showed the compounds retained over 98% of their Coulombic efficiency and 91% of their initial capacity over 46 hours of charging and discharging. ‘It’s a remarkably low-capacity fade of 0.15% per hour,’ the authors write.
However, further studies will need to look into the solubility of terthiophenes. ‘The solubility is very low compared to other redox-active molecules,’ says De La Garza. ‘This must increase to achieve competitiveness,’ adds Jorge Sobrido.
‘This organic molecule will never be close to a cost that’s feasible for a [commercial] technology,’ says Cedrik Wiberg, founder of Rivus Batteries, a company that manufactures redox flow batteries based on vanadium-free solutions. Nevertheless, he thinks researchers ‘have done a good job … investigating new molecular systems for energy storage’.
Bezdek recognises the limitations. ‘Although we haven’t yet assembled a fully functioning flow battery with our molecules, the fact that functionalised terthiophenes store electrons at extreme potentials is a promising proof-of-concept.’ They expect that further modifications to the structure will increase solubility, as well as energy density and robustness. ‘We’re keeping in mind the techno-economic realities of energy storage on scale [to] design the next generation of terthiophenes,’ he says.
References
D Kach et al, Angew. Chem., Int. Ed., 2023, DOI: 10.1002/anie.202304600















No comments yet