A small molecule drug candidate has been created that mimics a mechanism used by an antibody to fight flu. With the ever present threat of pandemic flu, as well as seasonal flu epidemics each year, the approach could boost the arsenal of anti-flu drugs while scientists continue to seek a universal flu vaccine that protects against all strains of the virus.
Influenza viruses evolve rapidly which can limit the effectiveness of vaccines and antiviral drugs. The 2009 global outbreak of swine flu and the looming risk of bird flu strains means there is an urgent need for a universal vaccine and new drugs that overcome resistance by targeting parts of the virus that remain largely the same, or conserved, between different strains.
One such conserved region of the flu virus is the stem of a mushroom-shaped surface protein called haemagglutinin. It’s the head of haemagglutinin that antibodies generated from seasonal flu vaccines usually target. Although accessible, the head is highly variable from strain to strain, hence the need for a new flu vaccine every year. The haemagglutinin stem, however, has remained a difficult target.
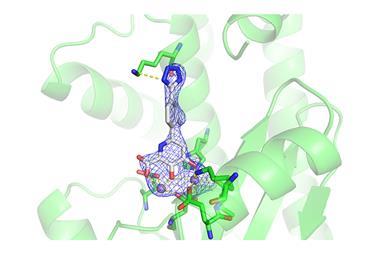
In recent years, broadly neutralising antibodies have been discovered that target the haemagglutinin stem and stop the virus fusing with cells, effectively locking out the virus particle and preventing infection. While this is a promising avenue for a universal vaccine, a research team has now successfully targeted the stem with an orally available small molecule drug that mimics the mechanism of a specific broadly neutralising antibody.
‘The strategy to use an antibody-assisted approach for the design of small molecules differs from the conventional medicinal chemistry approaches,’ says study co-author Ian Wilson at the Scripps Research Institute, US, whose lab collaborated on the molecule with drug firm Janssen. ‘Not only has the antibody information been invaluable for thinking about the design of more universal vaccines, but also for the design of broad acting therapeutics.’
The team developed the small molecule by first screening for compounds that target the same part of the haemagglutinin stem as a particular broadly neutralising antibody. The scientists arrived at a benzylpiperazine compound that neutralised flu infection in vitro. The molecule was then modified to improve binding and virus neutralisation, and further refined for stability and oral bioavailablity.
Mice were then given oral doses of the drug one day before being infected with a strain of swine flu (H1N1). The mice then received daily doses of the drug for a further seven days. After 21 days all these mice were still alive. By comparison, only half of the mice survived in a separate experiment with a less potent version of the compound. Further experiments revealed that the small molecule drug neutralised H1N1 infection in human lung cells too.
‘The study is an elegant example of rational drug design,’ comments Wendy Barclay, an influenza virologist at Imperial College London. ‘However, it described a dosing regime that includes a prophylactic dose. Although this might be a viable strategy during a large outbreak, it would be important to see how efficacious the drug is when given therapeutically at one or two days after infection as this is a much more realistic scenario.’
References
M J P van Dongen et al, Science, 2019, 363, eaar6221 (DOI: 10.1126/science.aar6221)










No comments yet