Synthetic overhaul for hepatitis C drug could ease manufacturing demands
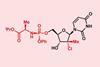
Chemists hope their five-step synthesis will increase global accessibility of uprifosbuvir and other lifesaving antivirals
A team at Merck & Co has developed a five-step method for synthesising uprifosbuvir with a 50-fold increase in yield over the previous manufacturing process. The scalable strategy minimises undesirable side products and could be adapted to synthesise a range of nucleosides to help meet global demand for antiviral therapies.