A thermodynamically stable example of the notorious ‘plumber’s nightmare’ structure has been created using a diblock copolymer by adjusting the interactions of the chain ends. The stability of the plumber’s nightmare structure – so called because the structure is so convoluted, an intricate tangle of tubes – is attributed to the interplay between the strength of the end–end interactions and the initial shape of the curvature. This work holds out the possibility of new ways to make other challenging materials.
Diblock copolymers consist of two types of monomers and can chemically self-assemble into nanostructures with different symmetries. As with any thermodynamic system there is competition between enthalpy and entropy which can be expressed as the minimisation of free energy. Phase diagrams of diblock copolymers have been established based on block polymer fraction, degree of polymerisation and the Flory–Huggins interaction parameter between the two blocks – a measure of the strength of interaction between the polymers. There are certain phases that are co-continuous in the equilibrium phase diagram; gyroid, double diamond and the plumber’s nightmare. And these are only stable over a narrow window on the phase diagram. These phases are interesting because they provide pathways in three dimensions without considering orientation, which is important for many types of applications including energy storage and soft electronics.
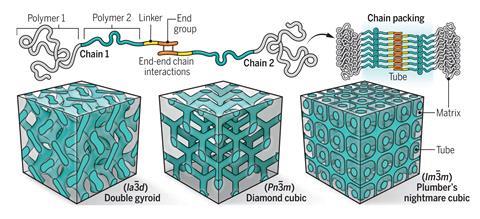
The plumber’s nightmare structure consists of a pattern of six intersecting tubes meaning that sample contains many tubes connecting and intersecting in complex ways. ‘Diblock copolymers have difficulty filling the space to form minimal [primitive] surfaces with six intersecting tubes compared with gyroid (three tubes) and diamond structures (four tubes),’ write the authors. Creating structures that are stable with high packing remains a challenge even though they have been hypothesised. The possibility of a thermodynamically stable plumber’s nightmare structure was predicted over 20 years ago ‘over a finite region of the phase diagrams, only when requirements for the composition or conformational asymmetry of the block chains are met’.
This led to the researchers investigating the thermal stability of the plumber’s nightmare structure in a diblock copolymer. They successfully created the structures by only modifying the terminal groups without changing the polymer backbones. The copolymer then self-assembled into the plumber’s nightmare structure when the researchers altered the temperature of the system. ‘This indicates that the [plumber’s nightmare to gyroid phase] transition is thermally reversible, and the [plumber’s nightmare] structure is in equilibrium at room temperature,’ the authors note. The importance of these findings is demonstrated by the consistency across two distinct type of block copolymers. These results prompt the revision of phase diagrams to consider the type of end–end interaction and linear chemistry to provide a method to assess other types of network structures with high packing frustration by controlling the arrangement and localisation of polymer chain ends. These highly unstable phases have become more accessible by simple tweaking of the chemistry where the chain ends have some enthalpic interactions that favour their coming together.
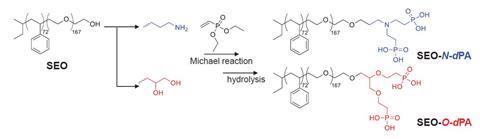
‘This is a beautiful study that has found a relatively simple way to overcome these free energy barriers to these higher order nodal structures… I’m sure that followers will use that [methodology] in order to take advantages of these phases for all kinds of applications,’ says Uli Wiesner, a materials scientist at Cornell University. He adds that these morphologies give significant benefits in the catalytic conversion of methane and CO2 to syngas, as well as altering superconductors properties ‘enabling applications all the way from separation to quantum materials as a result of these periodic lattices’.
References
H Lee et al, Science, 2024, DOI: 10.1126/science.adh0483















No comments yet