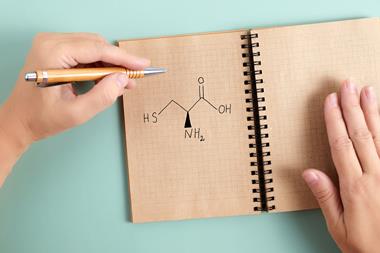
A sulfurous intermediate could be the missing puzzle piece that explains how simple molecules on early Earth first assembled into proteins. New research shows that aminoacyl–thiols can react with RNA molecules to initiate the first steps of protein synthesis, while avoiding competing side reactions, all without the need for enzymes.
How proteins originated is a longstanding paradox in biology. Today’s cells employ a two-step process called ribosomal peptide synthesis: the first stage loads an activated amino acid onto a molecule of transfer RNA (tRNA) that delivers it to the ribosome. There, a second step forms a peptide bond between the amino acid and a growing peptide chain that will eventually fold into a functional protein. Crucially, this sequence is heavily regulated by enzymes that must themselves have been created via protein synthesis – leaving an open question of how the very first proteins could have formed.
In particular, the initial activating step – an aminoacylation reaction – has proven challenging to replicate without enzymes. Previous studies have proposed various electrophiles including phosphates, imidazoles, and N-carboxyanhydrides as chemical activating agents but the resulting species are highly reactive, leading to uncontrolled background reactions and poor stability in water.
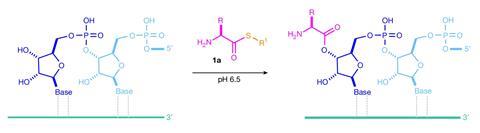
According to Matthew Powner and his group at University College London, UK, the solution must therefore involve a milder mechanism of activation. The researchers chose to focus their attention on thioesters, which are prevalent motifs in metabolic processes. Through a panel of experiments, they demonstrated that aminoacyl–thiols derived from from prebiotic precursors provided sufficient activation to enable these units to selectively bind to tRNA molecules. The water-based reaction seamlessly tolerated 15 different amino acid units while also suppressing the uncontrolled competing reactions of these amino acids directly with each other.
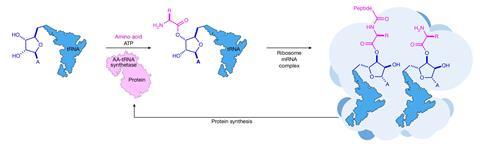
This directly parallels the role of ATP and synthetase enzymes in the modern process, explains Saidul Islam, an origin of life chemist at King’s College London who wasn’t involved in the work. ‘These amino-thioesters are at the Goldilocks zone of reactivity. It’s a very general process that works with all the amino acid side chains, and is taking place in water.’
In the presence of hydrogen sulfide, the thioester unit converts into a thioacid that Powner’s team showed could catalyse the second peptide-bond-forming step, providing the first template for enzyme-free peptide synthesis. For simplicity, they restricted this sequence to the formation of a single peptide bond but, proof-of-concept in hand, they are now working on expanding this to an iterative process to generate more complex polypeptides.
The team propose that the thioester unit could have derived from the reaction of amino nitriles with a sulfurous molecule called pantetheine. Earlier work by Powner and Islam has shown that pantetheine could have formed from simple chemicals that would have existed on early Earth. ‘[The new] work validates and underpins a lot of research that’s come before,’ says Islam. ‘You’re starting to see a cohesive story, a chemical lineage, where you’re going from very simple molecules to complex molecules.’
The next question to resolve will be how the genetic code began to control these peptide sequences. Ordinarily, the different amino acids are matched to tRNA units with a specific trio of nucleotides – known as a codon – which then associates that amino acid with a particular sequence in the genetic code. However, without synthetase enzymes to guide this pairing, there’s currently no clear link between the identity of the amino acid loaded onto each tRNA unit and the codon held on that tRNA within the thioester pathway. At present, this means that the peptide sequence is effectively random but Islam believes it’s only a matter of time until a relationship between these two components opens the gate to sequence-specific synthesis.
‘Overall, it’s an outstanding and thoroughly explored work,’ he says. ‘The beauty of origin of life research is that these ostensibly difficult problems are turning out to have quite simple and straightforward solutions if you just harness the natural reactivity of these molecules.’
References
J Singh et al, Nature, 2025, DOI: 10.1038/s41586-025-09388-y













No comments yet