Two new catalytic strategies offer ways to boost the Fischer–Tropsch process by preventing competing side reactions. The methods could improve the industrial process’s efficiency and reduce its environmental impact.
Since its discovery 100 years ago, the Fischer–Tropsch reaction has become a staple industrial process, combining hydrogen and carbon monoxide to generate hydrocarbons that can be used in the manufacture of many other valuable chemicals. However, the formation of water as a byproduct of the reaction consumes much of the hydrogen feedstock, while a complex series of parasitic side reactions converts up to 35% of the reaction’s carbon input into carbon dioxide. Now, two research teams in China have developed contrasting approaches to deal with these shortcomings.
Despite extensive study, the precise mechanism of the Fischer–Tropsch reaction remains elusive. It’s broadly thought that carbon monoxide dissociates into reactive carbon and oxygen species on the catalyst surface, followed by stepwise hydrogenation with atomic hydrogen (likewise generated at the catalyst surface). Subsequent carbon–carbon coupling, abstraction, or oxidation steps to form unsaturated or oxygenated products are less well understood and these productive transformations are unavoidably plagued by the water–gas shift and Boudouard reactions. ‘The water–gas shift reaction continuously generates carbon dioxide through water dissociation, while the Boudouard reaction directly disproportionates carbon monoxide, forming both carbon dioxide and surface carbon deposits,’ explains Ding Ma, a catalysis chemist at Peking University. ‘Together, these side reactions consume valuable carbon feedstocks, produce unwanted carbon dioxide and shorten catalyst lifetime.’
A hidden halogen
It was during a routine test experiment with an iron carbide catalyst that Xing-Wu Liu and Yi Cai, from Xiao-Dong Wen’s group at the Chinese Academy of Sciences, stumbled across a potentially transformative solution.1 The reaction had yielded unexpectedly low levels of carbon dioxide and subsequent spectroscopic analysis revealed trace levels of chlorine on the spent catalyst surface. ‘Suspecting halogen contamination in the syngas feed, we deliberately introduced different halogen-containing species and discovered that halomethanes, especially bromomethane, produced the most striking effect – almost eliminating carbon dioxide formation, while enhancing olefin production,’ recalls Ma, who collaborated on the project.
The team found that as little as 20ppm of bromomethane was sufficient to almost completely suppress the water–gas shift and Boudouard reactions. The researchers then used experimental techniques and computational models to elucidate a possible mechanism for the streamlined process.
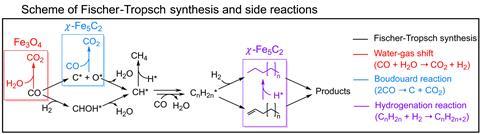
X-ray microscopy and spectroscopy revealed the catalyst surface was enriched in bromine and the group proposed that presence of these ions likely inhibits two key steps in each of the unwanted reactions. ‘Bromide inhibits water dissociation … cutting water–gas shift activity by more than two orders of magnitude; this removes the principal supply of [reactive species] needed to oxidise carbon monoxide into carbon dioxide,’ Ma explains. ‘[It also] prevents the recombination of carbon monoxide with surface oxygen – the key elementary step to form carbon dioxide.’
The near total suppression of carbon dioxide production, coupled with the impressive 85% selectivity for olefin products are an extremely encouraging advance, says Norbert Kruse, a catalysis chemist at Washington State University in the US. The next step will be to definitively confirm the role of the bromomethane in this mechanism, he adds. ‘This is conceptually new and exciting – one has to keep in mind … that halogens are usually considered poisons in heterogeneous catalysis!’
Despite lingering questions about the mechanism, this modification will likely be fairly straightforward to translate into existing Fischer–Tropsch processes, says Bert Weckhuysen, a catalysis researcher at Utrecht University in the Netherlands. Trials at scale showed that the catalyst was stable for more than 450 hours, while the team ‘underscored the broad applicability and robustness of the approach’ by applying the strategy to different iron phases, including a commercial iron catalyst, he adds.
A redirected side reaction
Rather than halting the water–gas shift reaction, Chaojie Cui and her team at Tsinghua University instead sought to harness the hydrogen it generates to drive olefin production.2 ‘Coupling the effect of the water–gas shift reaction and syngas-to-olefin reactions together, the hydrogen atom economy for producing hydrocarbons can be increased drastically, says Cui. She explains that this allows the use of feedstock with a lower hydrogen to carbon monoxide ratio.
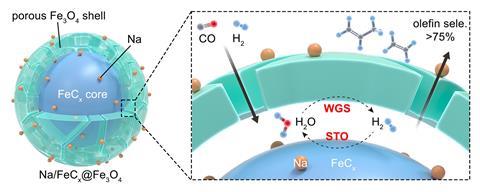
Cui’s team designed catalyst particles containing an iron carbide centre that promotes the syngas-to-olefin reaction, and a porous iron oxide shell that mediates the water–gas shift. Carbon monoxide and hydrogen therefore diffuse through the iron oxide pores, with the olefin production occurring in the cavity between the two catalytic layers. The water produced by this step then immediately undergoes the water–gas shift to regenerate hydrogen, still within catalyst cavity, maximising the incorporation of expensive hydrogen gas into olefin products.
‘This is a very nice spatial effect,’ says Weckhuysen. ‘The question is, can they make this at the scale of an industrial reactor and can they keep it stable over a long period, with methods for regeneration when it deactivates?’
However, Kruse believes that further analysis will be required to better understand the reaction mechanism and catalyst structure before the core–shell catalyst is developed further. Despite this, Kruse says that the efficiency gains demonstrated by both approaches are valuable contributions that could ultimately lead to more sustainable feedstock synthesis. ‘If we succeed in decreasing the costs for green hydrogen production, Fischer–Tropsch to olefins, as studied here, and oxygenates, as reported by others, could be designed entirely green, provided carbon dioxide formation can be avoided,’ he adds.
References
1. Y Cai et al, Science, 2025, DOI: 10.1126/science.aea1655
2. Gao et al, Science, 2025, DOI: 10.1126/science.aea0774
















No comments yet