Changing the chemical makeup of a polymer backbone could revolutionise how we make, use and even recycle plastics. James Mitchell Crow reports
For chemical species in the polymer kingdom, the backbone is their key defining feature. From naming and classification to properties and function, the backbone is central to what makes a polycarbonate a polycarbonate, a polyamide a polyamide, and a polyether a polyether.
A polymer’s backbone is the linear string of atoms, arranged in a regular repeating pattern, that forms the core of each polymer chain. ‘A big part of the identity of any polymer is in the backbone,’ says Alex Zhukhovitskiy, a synthetic polymer chemist from the University of North Carolina at Chapel Hill in the US.
The association with identity is perhaps partly why chemists haven’t tended to manipulate polymer backbones too much. According to the standard polymer production paradigm, once a polymerisation reaction is complete, the polymer’s backbone is set for the material’s lifetime. ‘There has been a lot of work on polymer modification over the years, but almost all of it has been focused on the periphery – the side chains and end groups,’ Zhukhovitskiy explains.
That position has recently begun to change and it’s now time for a concerted reassessment, Zhukhovitskiy argues in a recent review of the field. If a polymer’s backbone is the core to its identity, then modifying the backbone is essentially metamorphosing one polymer into another – an almost alchemic power.
The potential implications of this chemistry could be far-reaching. Mastering selective polymer backbone editing could change almost every aspect of the polymer lifecycle – from the way polymers are made to the functionalities they exhibit and to the ways they could be repurposed, upcycled and ultimately depolymerised at end of life.
‘Polymer backbone editing is an emerging area with a lot of potential to have a big impact,’ says Karen Wooley from Texas A&M University in the US. ‘People are getting excited about it, bringing in new ideas and it’s now just at the precipice, ready to take off and really fly.’
The chain
For Zhukhovitskiy, the idea of polymer backbone editing was inspired by several adjacent research fields, he says. ‘Probably the biggest influence was hearing Jennifer Doudna talk about Crispr DNA modification,’ he says. ‘Crispr is this absolutely remarkable way to edit backbones of biological polymers. As somebody with a synthetic polymer background, I started to think a lot about transforming abiotic polymeric backbones.’
A second source of inspiration came from the small molecule synthetic organic chemistry community. ‘When Mark Levin, Richmond Sarpong and others started talking about skeletal editing, there was this nice confluence of editing terms,’ Zhukhovitskiy says. ‘In my group we thought, why don’t we use this nomenclature for polymers, and start exploring this field – just as a fundamentally interesting way to modify polymer systems.’
The branding of a research field can be a double-edged sword, Zhukhovitskiy acknowledges. ‘It can be very distracting if everybody comes up with different ways of naming the same thing.’ But the right term can change the perspective from which you think about a problem, he says. ‘I think the editing terminology invokes selectivity and precision, in analogy with editing text where you can insert letters, delete them or change their order.’ Reframing a research area in this way can also serve as a focal point, concentrating renewed research effort, he adds.
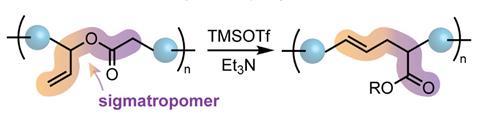
In 2021, Zhukhovitskiy’s group published their first examples of polymer backbone editing. They developed a rearrangement reaction in which atoms in the side chains and backbone of a polyester switch positions. ‘We took polymers that have ester linkages, and chemically treated the polymer in such a way that the atoms rearrange, and it becomes a polymer that has only carbon atoms in the backbone,’ Zhukhovitskiy says.
In this sigmatropic rearrangement, the backbone ester functionality switches places with a vinyl side chain, to form a polymer with an alkene in its backbone instead. ‘It becomes a vinyl polymer, which you would usually think of making in a completely different way,’ Zhukhovitskiy says. The team has also demonstrated a related polymer rearrangement reaction to bring a side chain oxygen atom into the backbone.
Accessing new polymer backbone structures as a way to enhance and diversify polymer properties is one of the key motivators for developing this chemistry, Zhukhovitskiy says. ‘You could have a material that has one set of characteristics, do some edits and suddenly you have a material with completely different properties to the original one,’ he says.
Polymer properties are often closely linked to backbone chemistry, Zhukhovitskiy adds. ‘To give one example, the property called glass transition temperature – which determines when a polymeric material changes from being hard, brittle and glassy to being rubbery – is heavily dependent on how rigid the backbone is.’
A potential benefit of backbone editing as a form of post-polymerisation modification is that it could dramatically expand the range of polymer structures that we can gain ready access to, says Steven Craig, a synthetic polymer chemist at Duke University in North Carolina, US.
‘Historically, there have been just a few ways that we’ve had to go about making polymers at any kind of scale,’ Craig says. ‘It tends to be simple additions, ring-opening reactions or condensation reactions.’ As a result, the kinds of structures that can be found along polymer backbones – and thus the properties that the materials display – also tend to be relatively limited. ‘Critical polymer properties, such as the limits to which polymers can be stretched, are very much related to the specific bonding and structure along the backbone,’ Craig says. ‘Coming up with creative chemistry to get at that has real implications for polymer properties.’
Polymer backbone editing could also enable new, greener ways to make polymers, Zhukhovitskiy adds. If you could install the desired backbone functionality in a post-polymerisation editing step, rather than having to install it from the start, this would open the possibility for making particular polymers from more sustainable starting materials, or by more sustainable methods.
Break it down
Applying synthetic organic chemistry to modify polymer properties was what first drew Wooley to the field. ‘What I find exciting about polymers is being able to control matter – like any synthetic organic chemist wants to do – but on the macromolecular scale, making things that you could hold in your hands and recognise their properties and their function, and how that could be useful to everyday life.’
This idea you can transform the polymer backbone is conceptually different and really exciting
Polymer synthetic chemistry has become increasingly sophisticated, Wooley says. Sometimes these enhancements have been borrowed from small molecule chemistry, but sometimes – such as ring-opening metathesis reactions – they are pioneered by polymer chemists and then transferred the other way. ‘When I saw Zhukhovitskiy’s paper I thought, wow, this is amazing chemistry – and I think that’s what happens when someone comes into a field and brings something new,’ she says.
‘For so many years, we as polymer chemists have been thinking about changing the chain ends, changing the side groups – and the backbone was the backbone, it was meant to remain intact,’ Wooley says. ‘This idea you can transform it is just conceptually different, and really exciting to think of the possibilities.’
In Wooley’s lab, polymer sustainability is already a key focus of her research, and it is this aspect of the backbone editing concept that particularly inspires her. ‘A central focus of my group for the last decade has been working with natural products – sugars, amino acids – and building polymers out of them,’ she says. Most recently, the team has been harvesting these materials from black soldier flies, the larvae of which are being explored as a potential agricultural product. ‘The adult flies are just waste, so we have been harvesting their chitin, although we could also get fatty acids, nucleic acids, proteins, all kinds of things out of these flies.’
The team has copolymerised sugars with carbon dioxide to make polycarbonates. But as well as sustainable starting materials, the team also thinks about sustainability at the end of life. ‘We want these polymers to break back down to regenerate those natural products after the lifetime is complete – and with backbone editing, there’s lots of opportunity for that,’ Wooley says.
Currently, developing reactions to break polymers back down into their original monomers, ready for reuse, is very difficult, Zhukhovitskiy says. ‘This inability is dependent on the backbone composition,’ he says. ‘If the bonds in the backbone are exclusively bonds that are really difficult to break, it may be very challenging to chemically recycle a polymer like that.’ With backbone editing, however, you could potentially insert cleavable bonds all along the polymer chain, turning a material that can’t be readily depolymerised into one that can.
In Wooley’s lab, her natural product-derived polycarbonates have already demonstrated a propensity for backbone alteration and structural metamorphosis. ‘We were finding that when were we were doing ring-opening polymerisation of cyclic monomers, they would back bite and do a rearrangement, essentially,’ she says. As a result, the regiochemistry of the monomer insertion into the growing polymer backbone – and the final polymer composition – would shift. ‘I love this idea of “Can we later come in and do further backbone modification and skeletal editing?”’ Wooley says. ‘I think there’s a lot of creativity that can be exerted here.’
Core competency
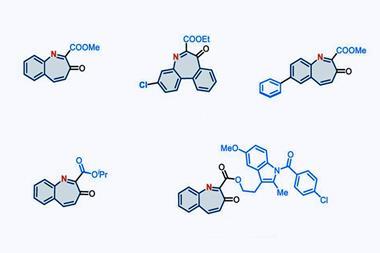
In his review article published in September 2023, Zhukhovitskiy and his group surveyed the polymer backbone modification field, ‘collecting and categorising examples of backbone editing scattered throughout a century’s worth of chemical literature, and outlin[ing] critical directions for further research’. The team hoped both to establish the foundations of the polymer backbone editing field, and accelerate its development.
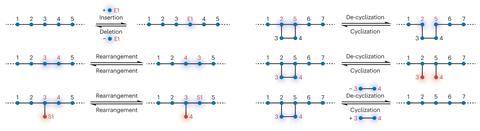
The examples of polymer backbone modification the team discovered in the literature included cyclisations, decyclisations, deletions, insertions – and combinations of the above. The earliest example they identified, a backbone editing reaction involving nylon 6,6 yarn, dates to a 1958 journal article by M R Porter from British Nylon Spinners Ltd.
‘That report was the one that surprised me the most,’ Zhukhovitskiy says. Using nitrogen tetroxide, acetic acid and sodium acetate, Porter converted nylon 6,6 – a polyamide – into a polyester. ‘Essentially, the reaction swapped the nitrogens in the backbone for oxygens,’ Zhukhovitskiy says. ‘It seems like a simple thing to do, but chemically one would be hard pressed to accomplish it – but they did it in 1958. Ideas like that were around even that long ago.’
The problem with the reaction – and the biggest challenge with polymer backbone editing across the board – is one of selectivity. For Porter, a competing polymer chain-scission side reaction resulted in an extreme fall in polymer molecular weight, from 10,000–12,000 to 300–500g per mol, as the chains got chopped up.
You can end up with some hybrid compositions with hybrid properties
‘One of the things that makes polymer backbone editing difficult is that polymers will record the history of what’s happened to them, in a way that small molecules don’t,’ Zhukhovitskiy says. If a small molecule rection proceeds with 90% selectivity, the 10% of molecules that underwent a side reaction can generally be separated and discarded. ‘But if a polymer backbone modification reaction works perfectly 90% of the time, and 10% of the time it misfires, there’s no way to separate those things from each other,’ he says. One in every 10 repeat units along the resulting polymer backbone will have the undesired structure.
‘If we’re trying to do polymer backbone editing, this high precision kind of transformation, then we would like to develop reactions that are as close to perfect as possible in terms of their chemoselectivity,’ Zhukhovitskiy says.
Achieving that level of selectivity is a challenge, Wooley agrees – but might not be necessary in every case, she argues. ‘A lot of people think you’ve got to get to 100%, but I think these intermediate stages of transformation are exciting opportunities,’ she says. ‘You can end up with some hybrid compositions with hybrid properties.’ There’s recent polymer precedent for this thinking, Wooley adds. For years it was thought very important that polymerisation reactions generated polymer chains of uniform length, she says. ‘But no – it has been recognised in the last decade or so that the skewing of the dispersity lets you blend properties of smaller and larger components, and a lot of times that leads to better performance,’ she says. ‘I think this partial skeletal editing might be an exciting area all on its own.’
Chemoselectivity doesn’t necessarily mean transforming 100% of the repeat units along the backbone – just that there is control as to where the edits occur, Zhukhovitskiy says. ‘The issue of site selectivity for where the transformation happens, how to direct transformations to particular repeat units, is a big challenge.’ Unlike DNA editing, there are no specific sequences to target, just a long string of identical repeating subunits to somehow differentiate between.
Purely from a fundamental polymer chemistry perspective, being able to modify specific sites along the polymer backbone – akin to the way specific positions on a small molecule skeleton can be modified, and the impact of this edit on its properties investigated – could generate new insights, Zhukhovitskiy argues. ‘There’s still a lot to be learned just about how to fundamentally tune material properties through specific changes in the backbone, because right now we don’t have a way of doing these edits.’
Partial editing may also have sustainability benefits, he adds. ‘When you are trying to upcycle a plastic, you might not need to transform every single repeat unit, all you may need is to transform 1%. That may be enough to, say, improve the adhesion of a polymer, to change its ability to form crystalline domains, to change its thermal properties.’
Pull factors
Polymer backbone editing may pose unique challenges compared to small molecule skeletal editing – but it also offers some unique opportunities. One area is in the use of mechanical force as a reaction trigger. Polymeric materials are inherently well disposed to mechanochemical approaches, says Zhukhovitskiy, who collected several examples of mechanochemically driven polymer backbone editing in his review. ‘You can think of polymer chains essentially as long lever arms, which can transmit the force and direct it to a particular site,’ he says.
What is the most drastic change we potentially could get in a polymer
Several mechanochemical polymer backbone editing reactions have originated in Craig’s lab. ‘The way we thought about it was “What is the most drastic change we potentially could get in a polymer and its behaviour in response to in situ mechanical forces?”’ Craig says. ‘If you could get the backbone to do crazy things like grow longer in response to these forces, that could hold some real implications.’
As a platform for developing mechanochemistry, polymers offer some distinct benefits. For one thing, you can exert the force simply by picking up a polymer and pulling on it. ‘The forces you can exert with your fingers are many, many orders of magnitude larger than the forces between atoms, and you can push and pull the atoms into places they would otherwise never think of going,’ Craig says. The team uses a variety of techniques to explore polymer mechanochemistry, including fluid flow systems and single molecule force spectroscopy.
Having multiple mechanophores installed along a polymer backbone also makes it easier to get a readout of the processes that occur, Craig adds. ‘It’s much easier to characterise 100 things happening on a backbone than just to characterise the single thing happening on a backbone.’
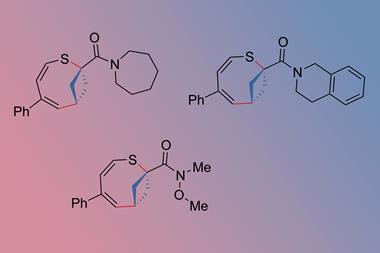
From a practical point of view, dramatic property changes can be accessed through applied mechanical force. Using force to promote a decyclisation reaction along a polymer backbone, a team at Stanford University, US, completely flipped the properties of a material called a polyladderene. ‘They converted an insulating polymer into a semiconducting polymer,’ Craig says.
In Craig’s own lab, he and his collaborators have used force-driven decyclisation chemistry to develop polymer backbones that could grow approximately 50% longer when force is applied. ‘Stretch them to the point where they would normally break, and now instead of breaking, they just grow half as large again as they started out,’ Craig says. ‘That ability to overstretch at the single molecule level translated to polymer networks that could absorb more energy than they were otherwise able to.’
The team’s work is inspired by applications where stretchy polymers mechanically wear down under repeated use. ‘I think about things like artificial heart valves, that eventually fatigue and fail, or the tread on car tyres,’ Craig says.
Craig was long motivated by images of tyre graveyards, vast mouldering dumps of old used tyres. ‘I always used to think of that as being the big environmental impact – but it turns out, it’s really the part of the tyre that you don’t see that maybe you have to worry about the most, all these little bits that have gone everywhere.’ Tyres are now recognised as a major source of microplastics, tiny rubber particles continually shed as they rack up the miles and slowly wear down. For electric cars, which are typically heavier than conventional cars and harder on their tyres, these microplastics are one of their biggest sources of pollution.
‘These little microplastics going off into the environment – that’s driven by chemical reactions, breaking bonds,’ Craig says. Polymer backbone editing may offer a way to modify these bonds to counter the problem, however. ‘Can I embed mechanically directed reactivity into polymer backbones in ways that change the mechanical outcomes that we typically associate with them?’ says Craig. ‘You can make strong arguments that there are ways you could completely rewrite the performance rules of polymers when you start thinking in that way.’
Molecules with a backbone have become some of the most dominant species in the chemical industry. Tyres are just one example of a material that is now ubiquitous in the modern world. ‘It’s hard to overstate just how vast the field is, and the implications of it,’ Craig says. ‘Polymers are now a half trillion dollar a year global business. If you think about the benefits that the industry has brought in terms transportation, biomedical safety, water safety – but also environmental impact - I think that context becomes pretty compelling.’
‘As a chemist, I think there’s a tremendous opportunity, now, for recognising the scale of a lot of these problems – but that there are opportunities that come out of really thinking molecularly and about chemical reactivity along polymer backbones.’
James Mitchell Crow is a science writer based in Melbourne, Australia













No comments yet