A new dual catalytic system can produce highly functionalised terminal alkenes from fatty acids. The technique could one day enable industrial production of valuable chemicals from a renewable source, such as vegetable oils.
Alkenes are one of the most useful classes of chemicals and can be turned into a range of products from polymers to pharmaceuticals. But most alkenes available in bulk are derived from petroleum-based materials. Now, a new method developed by researchers at the Max Planck Institute for Coal Research can produce terminal alkenes using waste vegetable oil as a starting material.
The new process uses a dual catalytic system involving a cobalt proton-reduction catalyst and an iridium-based photoredox catalyst to convert carboxylic acids into alkenes, with the loss of carbon dioxide and hydrogen. The process works for a range of different structurally complex carboxylic acids.
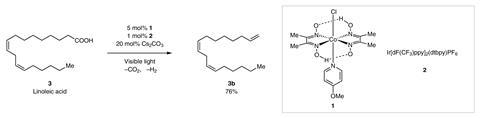
‘We have shown for the first time that you can access alpha olefins, that are really useful in large scale production of many materials, from carboxylic acids that grow – so it’s a renewable resource,’ says Tobias Ritter, who led the project.
The technique is the first homogeneous catalytic process to convert carboxylic acids into terminal alkenes without requiring stoichiometric amounts of additives. However, Ritter notes that the technique is still in development, a long way from being practical for large scale synthesis. For instance, the process relies on expensive precious metal catalysts, which would need to be made much more active for use outside of a research laboratory. ‘But at least conceptually we’ve shown that the process is viable,’ says Ritter.
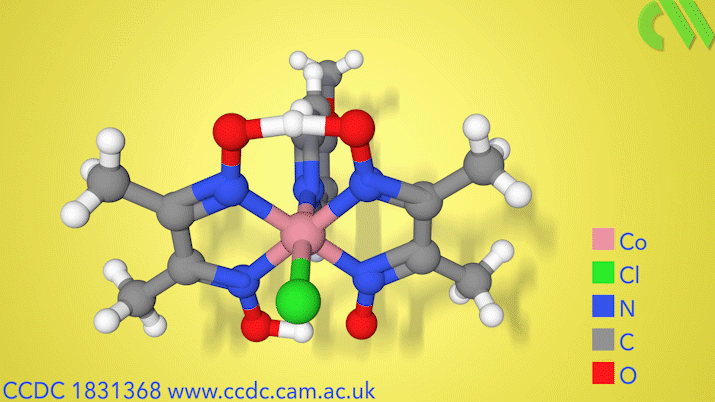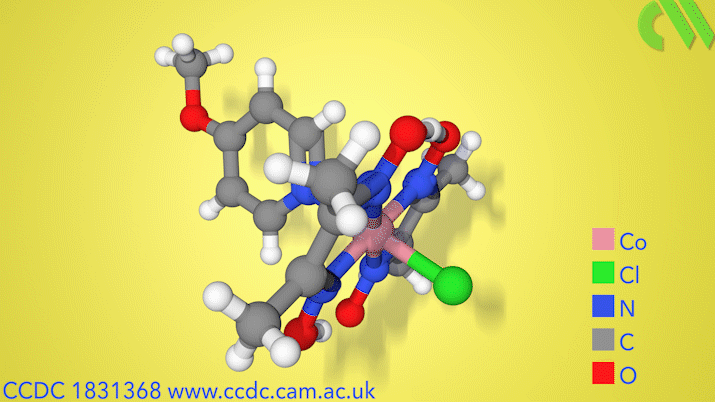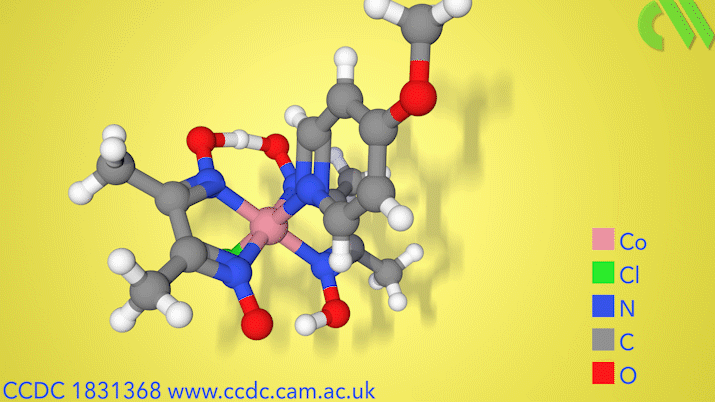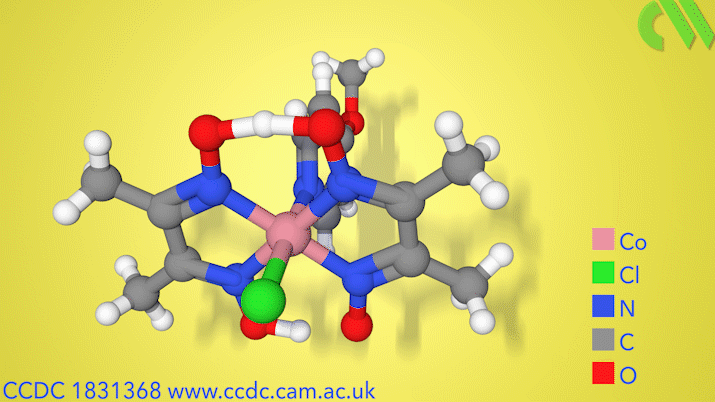
‘This bicatalytic system is quite nice, and that it can afford terminal alkenes with low loadings of base, is, as far as I know, unique,’ says Sergey Tin, who researches catalytic conversion of renewable materials at the Leibniz Institute for Catalysis in Rostock, Germany. Tin points out that other studies have previously produced internal alkenes from carboxylic acids catalytically, but notes that this is the first study to make useful terminal alkenes in such a way.
Ritter and his team now want to apply the technique more widely for late stage functionalisation of complex molecules. They also want to explore whether the catalytic system could be used to drive other types of reactions. ‘The interplay between the photoredox chemistry and the hydrogen evolution catalysis opens up a lot of possibilities that we can think about using in general synthetic chemistry, not just limited to making alpha olefins from acids,’ says Ritter. ‘So mechanistically there’s a lot more fertile ground.’
References
X Sun, J Chen and T Ritter, Nat. Chem., 2018, DOI: 10.1038/s41557-018-0142-4










No comments yet