Researchers in the US have developed a catalytic system for reducing carboxylic acids to aldehydes and alcohols in water.1 Critically, their system avoids the use of air and moisture intolerant reagents, precious metals and specialised ligands.
Many undergraduate courses in organic chemistry teach that carbonyl compounds can be reduced by hydride reagents such as lithium aluminium hydride and diisobutylaluminium hydride. While they are effective reducing agents, these compounds are notoriously incompatible with water and must be used under carefully controlled conditions. Modern chemists have therefore sought milder alternatives to these reagents, in line with the principles of green chemistry.
Now, Bruce Lipshutz and co-workers at the University of California, Santa Barbara have developed a catalytic system for reducing carboxylic acids in water via their thioester derivatives. The process converts aldehydes in a one-pot reaction with an organosilane reagent and a nickel catalyst. Alternatively, the thioesters could be converted to alcohols with sodium borohydride in 95% ethanol at room temperature. Crucially, they add a nonionic surfactant to the reaction mixtures that create micelles in which the reactions take place.
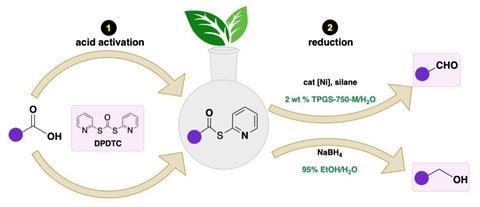
Lipshutz was inspired to develop the synthetic route in an effort to reduce his environmental footprint. ‘I had a bottle of a surfactant in water, almost pure, and we had been using it as an emulsifying agent for dietary supplements. It worked very well for putting things in water,’ explains Lipshutz. ‘I brought this surfactant down the hall to some grad students and a postdoc and said “Let’s put in substrates and catalysts and see what happens.” Well, everything worked – Heck reactions, olefin metathesis, Suzuki couplings – at southern California room temperature!’
The researchers investigated their new system with a range of substrates, including aromatic acids containing heterocycles (eg thiophene and pyrazine) and electron-withdrawing (eg nitro and nitrile) groups. They also screened some bioactive molecules such as naproxen and ibuprofen. ‘A clear substrate scope like this allows chemists considering using these conditions to quickly evaluate whether they are appropriate for their substrate,’ comments Dan Bailey, a senior scientist at Takeda Pharmaceuticals.
The researchers also calculated the E(nvironmental) factor of their reaction on a gram scale; the E factor is defined as the ratio of waste to product for a given reaction.2 ‘I was really impressed [that he] made a reaction in which the E factor was 2 – 2 is negligible!’ comments Luca Beverina, an organic chemist at the University of Milano-Biocca in Italy. ‘[A] standard organic transformation, for a scaled-up reaction, has an E factor on the order of anything between 50 and 100. E factors of 2 are something you see either when you make platform chemicals or in oil refinement where essentially there’s no waste.’
References
1 K S Iyer, C Nelson and B H Lipshutz, Green Chem., 2023, DOI: 10.1039/d3gc00517h
2 R Sheldon, Green Chem., 2016, 19, 18 (DOI: 10.1039/c6gc02157c)












No comments yet