This week, a versatile compound whose name is easily confused with the fictional metal fused to Wolverine’s skeleton in the Marvel universe. But comic book fans shouldn’t be disappointed – it may not be the marvellous metal of Logan’s claws, but it is the basis of a huge variety of medicines. Brian Clegg explains more…
Brian Clegg
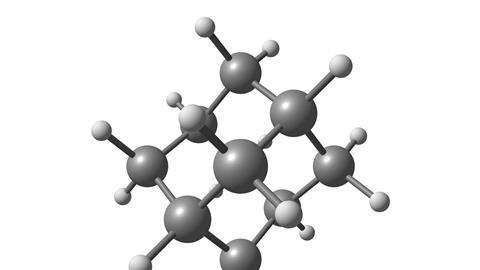
Fans of 70s and 80s music may be sad to learn that adamantane has nothing to do with Adam Ant, the ebullient stage persona of singer Stuart Goddard – nor is it directly connected to the adjective ‘adamantine’ meaning rigid and unbreakable; unlike the tough mineral adamantine spar, a form of corundum, adamantane isn’t particularly hard. Instead, the name adamantane could only ever have been a modern one, as it refers to the structure of this hydrocarbon, which resembles that of diamond, derived from Latin and Greek terms for extremely hard substances.

A colourless crystal at room temperature with a camphor-like smell, adamantane is an alkane with the formula C10H16, consisting of three linked rings, producing that diamond-like molecular structure. Although it visually resembles sugar, adamantane doesn’t dissolve in water – despite being easily soluble in organic solvents – and has an unusually high melting point for a simple hydrocarbon at 270 °C.
The compound was originally discovered in crude oil in 1932 by a process of fractional distillation, relying on the differing boiling points of the oil’s constituents. Adamantane is a very small part of the oil mix at no more than about 300 parts per million, so it wasn’t until the substance had been synthesised that it could be more than a passing oddity. Early methods of production involved complicated routes from larger organic molecules, but since the 1950s it has been produced from the naptha derivative dicyclopentadiene, in a two-stage catalysed hydrogenation and restructuring process.
Of itself, adamantane is structurally interesting, as the simplest of the so-called diamondoids – molecules that combine adamantane-like carbon cages – but it is not particularly useful. However, adamantane provides a starting point for the production of larger molecules, particularly medicinal drugs. A whole string of compounds have been built on the adamantane structure, starting in 1967 with adamantadine – simply adamantane with one hydrogen replaced by ammonia. This first proved effective as a flu antiviral and later was used in the treatment of Parkinson’s disease. Other derivatives have also proved effective antivirals, as they can block an ion channel – a chemical communication route in the cell that is used in replicating the virus and which ceases to function when blocked by the drug. Unfortunately, flu strains resistant to adamantane derivatives have become increasingly common.

However, tackling flu – or to be precise, the Influenza A virus – is just the beginning of the medical capability of adamantane derivatives. As antivirals they have also been used against herpes, hepatitis and HIV. Elsewhere they’ve treated acne, neurasthenia, Alzheimer’s, diabetes and malaria. The novel structure of adamantane, which is the hallmark of all its derivatives, gives it impressive properties. It has been described as a ‘lipophilic bullet’, because the structure seems to give the drugs just the right level of lipophilicity – dissolving in fats, oils and the like – to be effective, so much so that a 2013 paper in Chemical Review claims that apart from the methyl group, no other hydrocarbon structure has the same success rate in providing pharmacological activity. It is this same ability that helps adamantane-based drugs to cross the blood-brain barrier to work in neurological disorders such as Parkinson’s and Alzheimer’s. It has also been suggested that the cage-like structure could be used to hold other molecules for medical treatment which would then be released in the body as the structure breaks down.
Although non-medical applications are so far limited, there’s a strong feeling that adamantane’s molecular cage should have other potential uses, particularly in polymers containing repeated cages. Polymer structures with the cages along their edges have proved potential candidates for heat resistant substances, coping with temperatures up to around 370 °C, while epoxy resins containing adamantane cages could be valuable as the matrix used to hold LEDs. Other applications, from touchscreen coatings to high temperature hydraulic fluids, are being explored.

At first sight, adamantane is a disappointment. The name seems to promise something ultra-hard, capable of withstanding everything that nature can throw at it – when the reality is just a crystalline hydrocarbon. But adamantane’s ability to produce such a remarkable range of drugs means that, in its own way, it is a diamond of a compound.
Ben Valsler
Brian Clegg there on adamantane. Next week we’re cheating a bit, as the substance Katrina Krämer is bringing us is chemically interesting, though not technically a compound…
Katrina Krämer
The metal rolled around on the surface like a drop of water on a hot stove. But before it went transparent, it developed a deep blue ‘skin’ that quickly disappeared. This blue colour could only mean one thing: solvated electrons.
Ben Valsler
Find out how a classic classroom demo inspired cutting edge science next week, Until then, get in touch with any compounds you would like to hear more about by email to chemistryworld@rsc.org or on twitter to @chemistryworld. I’m Ben Valsler, thanks for joining me.












No comments yet