Meera Senthilingam
This week, a drug compound that changed the field of chemotherapy. Here's Jon Steed:
Jon Steed
Cisplatin is a tiny little drug molecule that contains a platinum ion in the middle of a flat square with two chloride ions and two ammonia molecules making up the corners. Approved for use in humans in 1978, it was the first of a completely new type of anticancer drug and remains an extremely effective and common treatment for conditions such as testicular and ovarian cancer.
But why on Earth would you want to put a heavy metal like platinum in your body? Platinum, like many heavy metals, is rather rare in the biosphere and as a result humans have not evolved to use it in our biochemistry, nor have we evolved any particular defence mechanisms against it. As a result, dosing yourself up on soluble forms of platinum results in some rather nasty toxic effects such as nausea and kidney damage (as the body tries to get rid of the stuff out of one end or the other), nerve damage and hearing loss.
The point, of course, is that cisplatin also fights cancer, and while partial deafness and nausea are not nice, they don't kill you like cancer does. Moreover, medical research has some excellent ways of minimising side effects and maximising the effectiveness of small doses of the drug.
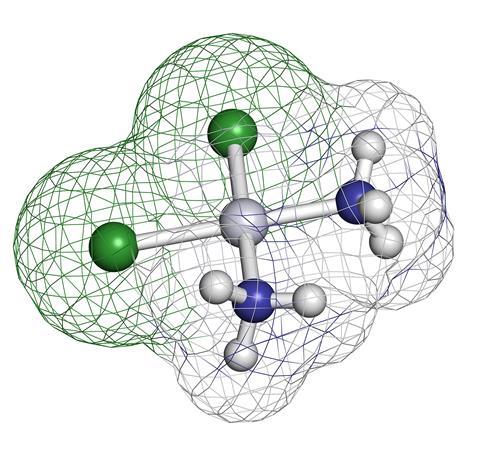
So why is cisplatin so good at fighting cancer? When cisplatin gets into the body, its neutral overall charge means that it can cross the cell membrane. Once in a cell it becomes activated by the replacement of one of the chlorides by a water molecule. The chloride falls off because the concentration of chloride within a cell is much less than it is in the bloodstream. The water itself is, in turn, easily displaced by the basic nitrogen atoms on DNA, specifically on a guanine nucleobase. Once bound to DNA the second chloride ion is replaced by a guanine nitrogen atom from an adjacent DNA strand.
The result is a platinum fragment cross-linking two DNA strands within the double helix. This cross-linking prevents the cell dividing by mitosis and so the tumour stops growing. The damaged DNA can be repaired in healthy cells by DNA repair enzymes, but in tumour cells the 'kink' induced by the platinum cross-link is not recognised and the DNA cannot be fixed. As a result the cell undergoes programmed cell suicide - apoptosis - and the tumour shrinks.
It is crucially important to use the cis form or 'isomer' of this square planar complex, where the chlorides are in adjacent, not opposite, corners of the square. Hence the name cisplatin. When the chloride ligands are replaced by DNA guanine the cis geometry is just right to fit in between the two double helix strands. The alternative isomer, transplatin, is not a useful drug and in fact is thought to become deactivated before it reaches the DNA.
While cisplatin is best known as an anticancer drug, the history of the molecule goes all the way back to 1845 when it was discovered by an Michele Peyrone - leading to the molecule being initially known as 'Peyrone's salt'. Its synthesis is a beautiful illustration of the chemical principle known as the trans effect.
Reaction of potassium tetrachloroplatinate with ammonia results in the initial displacement of one of the four chlorides to give a square planar molecule with three chlorides and one ammonia. To get cisplatin and not its trans isomer, where the chlorides are on opposite sides of the square, the second ammonia has to displace one of the chloride ligands adjacent to the first ammonia. Fortunately ammonia has a weaker trans effect than chloride which means that the first ammonia that binds is ineffective at pushing off the chloride ligand trans to it and hence forming undesirable transplatin. Instead one of the cis chlorides is lost and cisplatin is made.
The structure of cisplatin was unknown until the hugely important work of Alfred Werner in 1893 who developed the theory of coordination chemistry and showed that ammonia can bond to a metal ion like platinum +2 by donating its lone pair of electrons in a dative or coordinate bond. Werner's work was very much helped by the slow reactivity of square planar platinum +2 compounds which means that stay put long enough for chemists to study them.
Square planar platinum compounds have a distinguished history of beautiful experiments aimed at determining their structure. A classic demonstration was the platinum compound prepared by Mills and Quibell in 1935 involving cis ligands stilbene diamine and isobutylene diamine. The complex was designed to display a mirror plane if it had a tetrahedral structure but to be chiral if it proved to adopt a square-planar geometry. The fact that chirality was demonstrated using optical rotation data provided firm evidence for the square planar structure.
In 1965, a paper published in the journal Nature entitled 'Inhibition of cell division in E. coli by electrolysis products from a platinum electrode' led to the realisation that cisplatin inhibits cell division in bacteria. When treated with the compound, E. coli bacteria grow to 300 times their normal length but do not divide. The compound subsequently proved effective against sarcomas artificially implanted in rats and the medical career of cisplatin was born.
Cisplatin is the father of a number of other second and subsequent generation platinum anti-cancer drugs of which carboplatin and oxaliplatin with their more stable bidentate ligands and fewer side effects are perhaps the best known. These refined compounds are less harmful to the kidneys but are also less potent. Out of a few thousand platinum complexes tested over the past 40 years, around thirty have reached the clinic.
Research of platinum anticancer drugs has slowed down in recent years and there is a feeling that much of the 'low hanging fruit' has already been plucked. However, new candidates are still in the pipeline, particularly sterically hindered analogues such as picoplatin that aim to overcome increasing platinum resistance arising from binding with sulfur residues in proteins. There can be no doubt, however, that this simple, toxic coordination compound has saved the lives of thousands of people throughout the world and brought tremendous new insights into combating disease. Square planar it may be; but square and plain it 'aint!
Meera Senthilingam
Indeed, a drug compound that revolutionises the field of cancer therapy is certainly not square or plain. That was Durham University's Jon Steed, with the cancer-combating chemistry of cisplatin. Now, next week, a compound compound that we know has many uses, but that's still more prevalent in our daily lives than we may realise.
Brian Clegg
The industrial uses of salt go far beyond the culinary - in fact table salt accounts for just 4 per cent of our usage. It has long been employed to preserve food, absorbing water to dessicate the material, and features in industrial process from production of plastics to dyeing and the manufacture of detergents.
These days, if we spread salt on the land, it's most likely to be scattered as grit to prevent ice forming on roads, as a salt solution has a lower freezing point than water. Remarkably, around half the salt produced is now used on the roads.
Meera Senthilingam
And as well as the heavy use of salt throughout industry, join Brian Clegg to find out the discovery of this ubiquitous compound, its use as a weapon in war, and how far it's even infiltrated our everyday conversation in next week's Chemistry in its element. Until then, though, thank you for listening. I'm Meera Senthilingam.












No comments yet