All Atoms and bonds articles – Page 6
-
 News
NewsExplainer: why are curly arrows used in organic chemistry?
How organic chemists became arrow pushers and what quantum chemists have to say about this
-
 Research
ResearchControversy surrounds corrected chemical structures
Researchers used machine learning-powered NMR prediction to correct improbable structures – but some of their revisions have been challenged
-
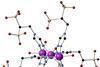 Research
ResearchSingle-molecule magnetic memory is the first to work at room temperature
Anionic iron complex breaks the rules of magnetic molecules and opens up opportunities to miniaturise data storage
-
 Research
ResearchThree-centre two-positron bond predicted
Findings expand group of molecules that combine matter and antimatter
-
 Research
ResearchChemical Turing machine reads molecular tape
Crown ether ratchet reads out molecular strand’s chirality
-
 Feature
FeatureWhen will molecular electronics make the connection?
Computer chips based on single molecules may remain a work in progress, finds James Mitchell Crow but the technologies developed along the way are being used by chemists to explore their reactions
-
 Opinion
OpinionNobel vision
Looking beyond the here-and-now let click chemistry open up a whole new world of possibility
-
 News
NewsQuantum technology pioneers win physics Nobel
Alain Aspect, John Clauser and Anton Zeilinger are honoured for establishing quantum information science
-
 Opinion
OpinionAre elemental analysis guidelines appropriate?
The standard journal requirement of ±0.4% for carbon, hydrogen and nitrogen is not statistically sound
-
 Research
ResearchBenzene’s bond lengths corrected
Sophisticated spectroscopic method shows that previously reported values were out by several milliangstroms
-
 Research
ResearchArtificial active transport offers new way to prepare complex oligorotaxanes
Novel class of molecular pump is driven by transamidation
-
 Research
ResearchTechnique can characterise actinides with just a microgram of a heavy element
Use of polyoxometalates offers chance to conduct in-depth research on heavy actinides chemistry
-
 Research
ResearchSuperheavy element flerovium is likely to be a liquid at room temperature
Element 114 predicted to be a volatile semiconductor with a melting point around 10°C
-
 Research
ResearchDiamond capsules allow ambient analysis of high-pressure samples
Crystalline forms of argon and neon can now be analysed using techniques that would previously have been impossible
-
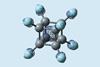 Research
ResearchPerfluorocubane catches electron in molecular box
Cube-shaped molecule can hold a single electron – a real-life version of the ‘particle in a box’ principle from quantum mechanics textbooks
-
 Research
ResearchElectrons become chiral reagent in polymer synthesis
Chiral polymer made from completely achiral chemicals using only electrons’ angular momentum
-
 Research
ResearchComputational study says polonium can form hydrogen bonds
Bonds driven by relativistic effects, rather than electronegativity differences
-

-
 Research
ResearchMetallic deuterium made at pressures rivalling those found at the centre of a planet
Synthesis could aid study of high-pressure superconductors
-
 Opinion
OpinionPeriod of discovery
Chemical space contained sufficient information to formulate the periodic system 25 years before Mendeleev