Calculations demonstrate that a stable molecule containing three hydride anions could be bound together by positrons rather than electrons.1
A positron is the antimatter analogue of an electron, possessing the same mass but a positive charge. The idea that a chemical bond could be mediated by positrons has been proposed before for the case of two hydride anions, where one or two positrons stabilise nuclei that would normally repel each other.2,3 Despite this, the nature of the positronic bond and the extent that it resembles conventional electronic bonds is unclear.
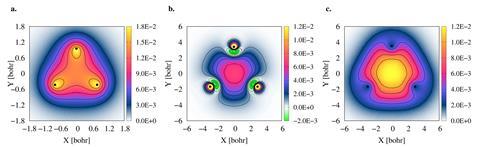
Andrés Reyes’ team at the National University of Colombia uses quantum Monte Carlo simulations to find compounds containing positronic bonds. In 2019 they reported that positronic covalent bonds between halide anions would be energetically stable.4 Now they have explored the stability of 2e+[H33−], a molecule that could hypothetically exist where three hydride anions are bonded by two positrons. Their calculations show that the bonding densities in the molecule are comparable to the isoelectronic trilithium cation, Li3+, which has an analogous bonding structure with three nuclei and two bonding electrons. They speculate that 2e+[H33−] might have metallic character like the trilithium cation.
In future studies the team hopes to show that positronic delocalisation, like conventional electron aromaticity, could exist in even larger positronic systems.
References
1 J Charry et al, Chem. Sci., 2022, DOI: 10.1039/d2sc04630j
2 J Charry, M T d N Varella and A Reyes, Angew. Chem., Int. Ed., 2018, 57, 8859 (DOI: 10.1002/anie.201800914)
3 D Bressanini, J. Chem. Phys., 2021, 155, 054304 (DOI: 10.1063/5.0059721)
4 F Moncada et al, Chem. Sci., 2020, 11, 44 (DOI: 10.1039/c9sc04433g)








1 Reader's comment