Philip Ball looks at the thermoelectric materials that harness waste heat and turn it into useful energy
Fed up with rising petrol prices? You’ll probably feel a good deal worse about them once you know that most of the costly fuel’s energy content is wasted. In a conventional car with an internal combustion engine, only about a third of this energy goes into propelling the vehicle down the road. The rest is squandered as heat.

Scientists and engineers have been trying for decades to capture waste heat like this – not just in vehicles, but in factories, electronic circuits and natural heat sources – and do something useful with it. One of the most promising avenues uses thermoelectric (TE) materials, which have the marvelous ability to turn heat into electrical current. But these efforts have been hampered by the fact that TE materials are inefficient, expensive to make and often contain toxic elements.
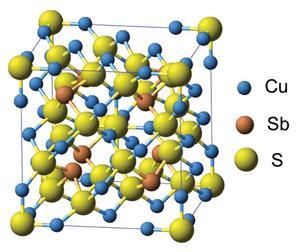
Towards the end of 2012, two teams based in the US and Japan independently discovered that, rather than trying to develop new and complicated formulations for a good TE material, we can just dig one out of the ground. They reported that compounds related to a mineral called tetrahedrite – a copper antimonide sulfide, the most widespread complex sulfide on the planet – can show thermoelectric performance that rivals any of the materials currently under consideration.
The discovery ‘really captured my imagination’, says James Salvador, who is working on such systems at the General Motors laboratories in Michigan, US. He recalls once half-joking with a colleague that for a TE material to be viable for cars, it shouldn’t cost much more than sand. ‘Then along comes this mineral that you can literally pull right out of the ground, make a few minor compositional changes to it and you get a darn good TE material,’ he says. ‘It makes you wonder what else might be sitting out there just waiting to be discovered.’
Hot power
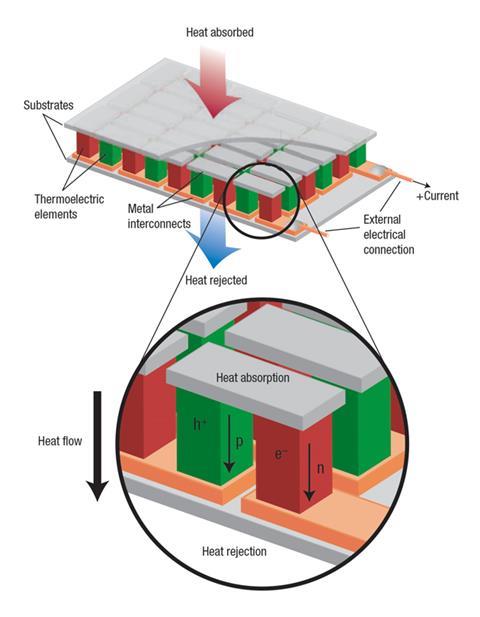
Conversion of heat to electricity by thermoelectric modules is already exploited in applications where cost is less of a factor: pellets of radioactive material, heated red-hot by their decay processes, power the electrics in all deep-space probes, such as the Cassini mission to Saturn and the Mars rover Curiosity. But such systems must be much cheaper and more efficient if they are to make a big difference to the fuel efficiency of cars. The TE devices would sit around the car’s exhaust pipe and extract heat from gases discharged at around 300–500°C. Capturing just 5–10% of a vehicle’s waste heat could reduce fuel consumption by around 3–6% – a significant saving today. But the benefits are currently small relative to the cost. To make it worthwhile, TE materials need to get more efficient, and they need to be affordable. ‘There needs to be a significant improvement in performance to reach a tipping point that will make this technology a no-brainer solution,’ says Salvador.
That heat can be converted into electricity was discovered only shortly after electricity itself was harnessed for scientific study in the late 18th century. In 1821, Thomas Seebeck, a Prussian scientist born in what is now Estonia, discovered that when he joined the two ends of a copper wire to those of a bismuth wire and heated one of the junctions, an electrical current flowed through the loop. Seebeck thought that this was a magnetic effect – the connection between electricity and magnetism was just then emerging from the work of Humphry Davy and Michael Faraday. Twelve years later, the French watchmaker Jean Peltier observed the reverse effect: a current passed through a junction of two metals could heat it (beyond simple resistive heating) or cool it.
From the early 20th century, these so-called Seebeck and Peltier effects were used to make electrical generators and heat pumps. But it was only in the 1950s that the technology began to take off, when researchers started to look beyond metals for thermoelectric effects. It was discovered that both bismuth and lead tellurides had greater thermoelectric efficiency, and these compounds have been the mainstay of the field until recently, when clever materials chemistry has started to reveal a whole unexplored vista of candidates.
Phonon filters
If a temperature gradient exists across a material, the charge carriers – electrons and the electron vacancies called holes, which behave like positively charged particles – will diffuse preferentially from the hot side to the cool side. If the material has more electrons than holes, or vice versa, this diffusion will set up an imbalance in charge between the hot and the cold side: a voltage that can be tapped to extract electrical current.
The voltage produced by the Seebeck effect is generally proportional to the difference in temperature between the hot and the cold junctions. The constant of proportionality (Seebeck coefficient) is a measure of how efficient a thermoelectric material is at making electricity from heat. But there are other factors that determine its performance too: ideally the material must be a good conductor of electricity but a poor conductor of heat. All these factors are rolled up into a ‘figure of merit’ for thermoelectric performance denoted zT, where T is the temperature at which the device operates. Conventional telluride thermoelectrics have a zT of around 1. To increase the efficiency, and thus the mileage, of an internal combustion engine by 10% would require a zT of around 1.25 for a typical exhaust temperature of 350°C. But to make many such applications commercially viable, many researchers agree that a zT approaching 2 is needed.1
Materials that conduct electricity well also tend to conduct heat well – just think of metals. So how do you get one without the other? That means impeding the motion of the atomic-lattice vibrations (phonons) that transmit heat, while not obstructing the motion of the mobile electrons or other charge carriers that make up the electrical current.
Phonons have wavelengths of typically a few nanometres to a few hundred. In the 1990s, it became understood that phonons might be hindered by making a material structured at the same kind of scale so that the phonons are strongly scattered, just as sub-micrometre globules of fat in milk are the right size to scatter light and make the liquid opaque.2 The traditional TE materials, lead and bismuth tellurides, can also be improved by introducing phonon-scattering defects in the crystal structure: gaps (vacancies) or misplaced atoms (interstitials). This kind of trick was already being unwittingly explored in the 1950s using complex alloys of metals and semimetals with dizzying formulas such as (AgSbTe2)0.15(GeTe)0.85 and (AgSbTe2)x(PbTe)1–x, in which nanoscale crystals of one phase were probably precipitated in an uncontrolled way within a matrix of another.
Tailor-made phases
More recently, the engineering of nanoscale grain boundaries has become a rather precise art: for example, materials chemist Mercouri Kanatzidis and his colleagues at Northwestern University in Illinois, US, have reported zT values of about 2 from nanostructured forms of lead telluride, selenide and sulfide doped with other metals.3 Achieving such high performance in lead telluride is ‘a dream come true’, Kanatzidis says. ‘No one imagined this even 10 years ago.’
Compositional complexity can also give rise to large unit cells or local disordering that can impede phonons.4 One popular class of TE materials currently being explored are the so-called filled antimony skutterudites. Skutterudite itself is the mineral cobalt arsenide; the equivalent antimonide CoSb3 has a crystal structure made up from CoSb4 tetrahedra joined at their corners. This creates a rather open framework that can be ‘filled’ with rare-earth atoms such as cerium and lanthanum, which rattle around in a disorderly way. Much of the work on filled skutterudites has focused on ternary compounds in which some of the cobalt atoms are substituted by iron.
No one imagined this 10 years ago
Mercouri Kanatzidis
Another class of promising complex alloys is the so-called half-Heusler phases, which are either nickel–tin or cobalt–antimony compounds containing early transition metals such as titanium, zirconium and hafnium: they have formulas such as Hf0.75Zr0.25NiSn. These too have been nanostructured to improve their performance, for example by incorporating nanoscale inclusions of metal oxides through a mechanical milling process, or by making nanocomposites of layered thin films.4 Seldom has materials engineering seemed more like a sophisticated version of making a cake – whether it’s a fruit cake, a layer cake or a marble cake.
Skutterudites and half-Heusler phases have been the mainstay of much of the recent work on TEs, although there’s now also interest in alloys of magnesium or manganese with silicon and tin. Kanatzidis and colleagues have recently reported5 a zT of 2.6 at the rather high temperature of 650°C along one crystal axis of single-crystal tin selenide (SnSe). But while zT values have been creeping up, reproducibility can be a challenge for such complicated mixtures, alloys and microstructures.
Besides, says materials physicist Zhifeng Ren at the University of Houston in Texas, US, record-breaking efficiencies are all very well, but cost is still the most important factor determining a TE material’s potential utility. Right now, he says, there is no ideal system that meets all the criteria of high efficiency, high power output, stability and low cost. ‘It is always good to improve the existing materials, since we know more about them,’ he says. ‘But it’s even better to find new materials with much better properties.’
The power of rock

That’s just what happened with tetrahedrite. These minerals are mixed antimonide-sulfides with the general formula Cu12–xMxSb4S13, where M is a transition metal such as zinc or iron. This means that they contain no toxic elements like lead. What’s more, mineral tetrahedrite costs as little as $4 (£2.40) per kilogram. Other leading candidate materials can cost up to 40 times as much.
Donald Morelli of Michigan State University in the US and his coworkers discovered the very promising TE properties of such compounds after theoretical calculations tipped them off – it was ‘an unexpected and exciting bonus’, says Morelli, that they turned out to occur naturally in the form of tetrahedrite.6 The mineral form itself doesn’t have the ideal composition, but it’s simple to adjust it by mixing the ground-up mineral with the right amounts of powdered copper, sulfur and antimony and hot-pressing the mixture into pellets.7 Alternatively, the stuff can be made from scratch using the appropriate quantities of the respective elements. With the optimal composition, the tetrahedrites can achieve zT values of around 0.9, comparable to state-of-the-art single-phase lead telluride.
At much the same time a group working at the Japan Advanced Institute of Science and Technology (JAIST) in Ishikawa, led by Koichiro Suekuni, stumbled on the same solution while trying to synthesise an artificial TE material containing copper and sulfur.8 ‘I was inspired by earlier studies of sulfide TE materials,’ Suekuni explains. His investigations also brought him to crystallographic studies of tetrahedrite, and the group found that the nickel-containing version can display a zT value of around 0.7 at 400ÀC. They attributed this behaviour to unusually large-amplitude vibrations of the copper atoms, which seem to disrupt heat transfer and give the material such a low thermal conductivity.
Although it’s not hard to make these materials synthetically in the lab, they would be affordable only if that can be done cheaply enough. ‘If samples made from low-purity, low-cost materials show similarly high performance as those made from high-purity elements, mass production of the tetrahedrites would become possible’, says Suekuni. But in natural tetrahedrite the local composition varies from place to place, so he cautions that ‘it’s too early to know if we can obtain high-performance materials in large amounts from the natural ones’. All the same, Morelli and colleagues managed to get pretty good performance from samples made by mixing natural and synthetic ingredients.6
In July 2014, Morelli completed an agreement with the California-based company Alphabet Energy to commercialise tetrahedrite-type compounds for TE applications. Alphabet has also been collaborating with researchers at the Lawrence Berkeley National Laboratory in California, led by mechanical engineer Arun Majumdar, who are developing TE devices based on silicon nanowires.9 At the moment Alphabet is seeking to develop stand-alone TE modules, although it is also working on devices that could harvest heat from car exhaust.
Making it work
Even with this recent boost, can thermoelectric devices ever achieve the performance and cost needed to make the capture of waste and low-grade energy routine? ‘For a long time the performance was always just on the edge of what might work,’ Kanatzidis says – but now he thinks that the potential for significant fuel savings is really becoming apparent. But Salvador is more cautious. ‘We need to be honest with ourselves,’ he says. ‘Even with the strides the community has made with materials and systems development, the efficiencies are still pretty low.’ They’re also not so robust against shocks and extreme vibration. For this reason, Salvador says, ‘the automotive industry is not that close to getting waste heat recovery devices in a vehicle – we’ll need better materials before this becomes applicable to a broad range of vehicles’.
However, it’s possible that anti-pollution legislation might help to tip the economic balance – at least in Europe, where new legislation dictates that, from 2020, if the average mileage of passenger cars is not above about 70 miles per gallon then carbon-emission penalties will boost the cost of the vehicle. Mindful of such constraints, Volkswagen, BMW, Daimler-Benz, Ford and other car manufacturers are now developing prototype TE systems.
‘The thermoelectrics community seems to be at a turning point’, says Salvador. ‘We are seeing a lot more emphasis on components and systems development than we did even five years ago.’ Some small companies are already introducing TE waste-heat recovery systems for stationary industrial settings. ‘In the industrial settings you have a lot of waste heat that is pretty high grade, the equipment is on all the time, and the payback period under those conditions looks a lot more favourable,’ says Salvador. To turn the corner for cars, there will be more engineering of the elements yet to be done.
Philip Ball is a science writer based in London, UK
References
1 L E Bell, Science, 2008, 321, 1457 (DOI: 10.1126/science.1158899)
2 G Chen et al, Int. Mater. Rev., 2003, 48, 45 (DOI: 10.1179/095066003225010182)
3 K Biswas et al, Nature, 2012, 489, 414 (DOI: 10.1038/nature11439)
4 G J Snyder and E S Toberer, Nat. Mater., 2008, 7, 105 (DOI: 10.1038/nmat2090)
5 L-D Zhao et al, Nature, 2014, 508, 373 (DOI: 10.1038/nature13184)
6 X Lu et al, Adv. Energy Mat., 2013, 3, 342 (DOI: 10.1002/aenm.201200650)
7 X Lu and D T Morelli, Phys. Chem. Chem. Phys., 2013, 15, 5762 (DOI: 10.1039/c3cp50920f)
8 K Suekuni et al, Appl. Phys. Expr., 2012, 5, 051201 (DOI: 10.1143/apex.5.051201)
9 A I Hochbaum et al, Nature, 2008, 451, 163 (DOI: 10.1038/nature06381)











No comments yet