How the party drug ketamine may hold the key to treating patients with severe depression
One in four people in the UK experience a mental health problem each year, according to mental health charity Mind. And while the majority improve with time, exercise, talking therapies or antidepressants, a significant number do not. For these so-called treatment-resistant patients, the landscape of alternative clinical options is bleak.
Help may now be on its way from an unlikely source: ketamine. Recreational drug users have long reported an ‘afterglow’ following its use, with positive mental effects lasting for hours after the drug’s dissociative effects have worn off. This phenomenon sharply contrasts hangovers from alcohol.
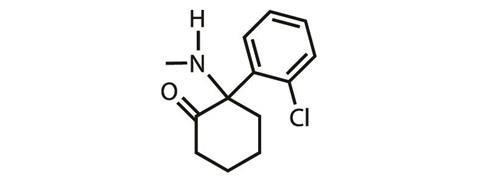
Ketamine was first approved for use as an anaesthetic in the US in 1970, and is now licensed as an anaesthetic all over the world. As early as the 1970s – as its popularity grew through new age culture and the club scene – ketamine’s antidepressant capabilities were recognised, explains Steven Levine, a psychiatrist and founder of the Ketamine Treatment Centers in the US. ‘The field of psychiatry has danced towards it [ketamine] and away from it over time,’ he adds.
But over the last decade efforts to explore its potential as an antidepressant have gained momentum. There are two branches to this work: its off-label use for treating severe depression in clinics such as Levine’s, and the development of novel drugs designed to mimic the way ketamine works in the body.
In the clinic
Spurred on by positive small-scale government and academic trials, in 2011 Levine opened his first Ketamine Treatment Center in Princeton, New Jersey. Here, depressed patients who have not responded to any available treatments are given a very low dose of ketamine intravenously. Those patients that respond positively receive six infusions over two to three weeks, followed by a single maintenance treatment approximately once a month.
Levine now runs seven treatment centres across the US, and estimates that 20 other private and academic clinics have opened recently offering ketamine infusions. As an off-label treatment, ketamine infusions are not covered by patients’ health insurance, but the mushrooming of these clinics is a strong indicator that patients think ketamine is worth paying for on their own.
Ketamine offers a rapid antidepressant effect in people who’ve otherwise been depressed for many years
Levine estimates that 70% of his patients respond to ketamine infusions in his clinics, and similar success rates have been consistently reported by other clinicians. But ketamine offers even more than this: ‘It can work within hours of a single treatment and you don’t need to maintain a blood level of the medicine,’ Levine explains. ‘You also don’t have any side effects in between treatments.’
By contrast, the conventional antidepressants, selective serotonin reuptake inhibitors (SSRIs, such as fluoxetine) and serotonin-norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine) can take up to eight weeks to take full effect, do not work for millions of patients with depression, and have a substantial list of potential side effects. ‘Here we have a medicine that works completely differently, and the expectations for when and how it works are completely different. It really is a complete paradigm shift,’ says Levine.
‘Ketamine works by different mechanisms to conventional monoamines and offers a rapid antidepressant effect in a significant portion of people who’ve otherwise been depressed continuously for many years,’ agrees Rupert McShane, a consultant psychiatrist at University of Oxford, UK. In 2014, McShane reported positive results of the first UK study of intravenous ketamine for treating people with severe depression.
Since this funded study ended, McShane has opened the UK’s first private clinic offering ketamine infusions to patients who are willing and able to pay for the treatment. In total, about 100 patients have been treated with around 1000 infusions of ketamine, he says.
Esketamine
As the mountain of evidence grows that ketamine is an effective medication for ‘treatment-resistant’ depression, one might assume that it could soon be licensed for this indication. But this isn’t the case. ‘Ketamine is an old, generic drug and will be unlikely to get FDA (the US Food and Drug Administration) approved [for depression],’ explains Levine. To gain approval in the US and most other countries, large-scale clinical trials would be needed, costing millions of pounds. ‘The only reason why somebody would do that is if they had a financial interest in it.’ And a drug that is already off patent will never earn a company back those big bucks.
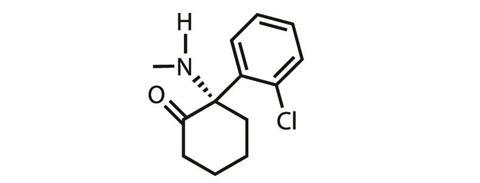
But ketamine is a racemic drug, and Johnson & Johnson (J&J) subsidiary Janssen Pharmaceuticals is now moving forward with gaining approval to use the S-enantioner (known as esketamine) to treat depression. ‘They have secured the rights for a use patent on the intranasal delivery of esketamine, a drug that has been approved in Europe for 15 years [as an anaesthetic],’ explains Levine.
Intranasal esketamine against treatment-resistant depression is currently in Phase III clinical trials in the US and over 20 other countries. (McShane’s is one of the participating clinics in the UK.) ‘We’re currently doing five large Phase III international multi-centre trials,’ explains David Hough, a psychiatrist and esketamine team leader at Janssen R&D in Titusville, New Jersey, US. They are planning to file for approval for this indication in 2018, he adds.
Like ketamine, Janssen intends that esketamine will be used in a clinic, although theoretically an intranasal medication could be taken at home. ‘There are some short-term side effects that we believe need to be monitored by a healthcare professional,’ Hough says. Even at these very low doses, esketamine can cause short-term dissociative side effects. ‘We also want to make sure that there’s not potential for abuse liability.’
Janssen are also trialling intranasal esketamine against a second indication: major depressive disorder with suicide ideation. ‘These are patients who are at imminent risk for suicide,’ says Hough. It is the rapid patient response to ketamine that is so important here. Phase II trials against this indication are now complete, with Phase III trials planned to start in 2017.
It’s worth noting that neither intravenous (IV) ketamine nor intranasal esketamine are intended to be used as stand-alone treatments. ‘Whatever oral anti-depressants the patient’s been taking they will continue, and this will be a supplemental, or adjunctive, treatment,’ Hough explains.
Rapastinel
Ketamine is well known to block activity at NMDA (N-methyl-D-aspartate) receptors and some drug companies are looking to develop novel drug molecules that work on the same receptor, whilst simultaneously hoping that targeting the receptor differently will eliminate the psychotomimetic side effects seen with ketamine itself.
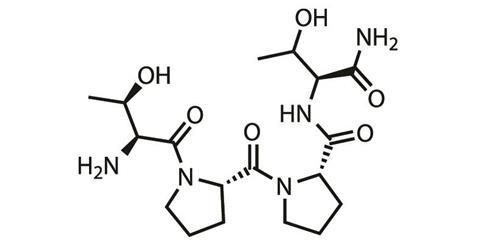
One such company is Allergan, headquartered in Dublin, Ireland. Allergan has a peptide, rapastinel, in Phase III trials in the US as an adjunct therapy for the treatment of major depressive disorder. ‘We will be doing studies in Europe and also, hopefully, in Japan as part of the global development programme,’ says David Nicholson, chief R&D officer at Allergan.
‘Rapastinel works within hours and a single administration gives a long duration effect,’ he says. ‘This is similar to the many studies that are being done with ketamine for depression.’ However, it differs from ketamine in its specific action at the NMDA receptor, but exactly how is still being figured out. Ketamine blocks NMDA receptor channel activity, while rapastinel is suspected to modulate and potentially enhance NMDA receptor activity. In trials so far, rapastinel has shown efficacy against depression while having a very low rate for dissociative effects.
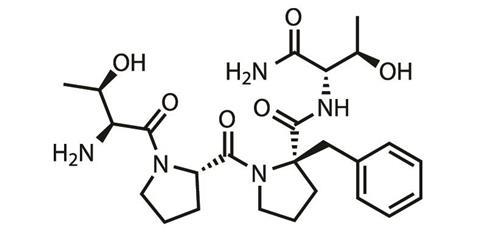
Rapastinel is to be given intravenously in clinics and to broaden its customer-base Allergan is looking for an oral drug that shares the same pharmacology. First up was apimostinel, another peptide, which struggled in Phase II trials due to low oral bioavailability. Allergen has now turned its attention to a small molecule with better oral bioavailability currently in pre-clinical development, says Nicholson.
AV-101
Another company looking to develop a small molecule drug that mimics all the good parts of ketamine, and none of the bad, is VistaGen Therapeutics. This biopharmaceutical company based in San Francisco, US, has an oral, small molecule drug (AV101, L-4-chlorokynurenine) in Phase II trials in the US against major depressive disorder.
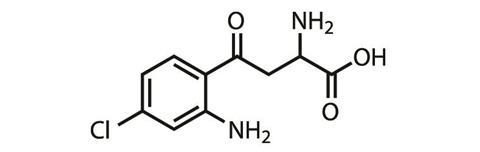
AV101 is a pro-drug, explains Mark Smith, a psychiatrist and chief medical officer at VistaGen. ‘The active part of AV101, 7-chlorokynurenic acid, is actually an old drug candidate from the 1980s,’ he says – one that struggled to get into the brain. The pro-drug, however, effectively crosses the blood-brain barrier, and is then metabolised into the active molecule. 7-chlorokynurenic acid behaves differently at the NMDA receptor to both ketamine and rapastinel, modulating its glycine binding site and acting as a full antagonist here.
‘Ketamine blocks NMDA channels like a cork in a bottle, plugging up the ion channel. AV-101 works more like a modulator, or a thermostat, on the NMDA receptor. It blocks the glycine site, and therefore inhibits NMDA function, but we think in a much gentler manner, which is perhaps why it has a better side effect profile than ketamine,’ explains Smith.
In rodents, AV101 has shown a rapid and persistent response like ketamine, but with a completely different side effect profile. It won’t be known until further into the clinical trial programme whether this is also true in humans. And while a small trial is currently underway using AV-101 as a standalone treatment, it is intended to ultimately be used as an adjunctive therapy similar to all the other drugs discussed so far. Following the lead of Janssen with esketamine, VistaGen is also starting to explore whether AV-101 could have some utility against suicide ideation.
NRX-101
Another company with an eye on suicide ideation is NeuroRx, based in Wilmington, Delaware, US. It is developing NRX-101 for acute suicidal ideation associated with bipolar depression. NRX-101 is a proprietary combination of two approved drugs: the antibiotic D-cycloserine, an NMDA receptor modulator acting at the glycine site, and the antipsychotic lurasidone, a 5HT2a receptor antagonist.
‘This dual mechanism approach is expected to provide antidepressant and antisuicidal effects while balancing out the dissociative effects, which can be caused by NMDA agents, and the restlessness that can increase suicidal thoughts, which can be caused by serotonin based drugs such as 5HT2a antagonists,’ explains NeuroRx chief commercial officer Robert Besthof.
The approach differs from the others in that NRX-101 is to be studied after initial stabilisation with IV ketamine, as a way to orally extend the effects of a ketamine infusion without the patient needing to return to a clinic for further IV treatment. Following a single ketamine infusion, patients will receive a daily dose of NRX-101 for approximately six weeks, explains NeuroRx chief commercial officer Robert Besthof. ‘We think that an oral regimen, with a drug that most likely will not be scheduled (since neither component is scheduled), might be much easier to use in the treatment of such a high-risk population.’
A Phase IIb/III trial is expected to start in the US in a few months. As with ketamine, NRX-101’s story started serendipitously following numerous anecdotal reports of patients feeling less depressed after taking D-cycloserine as an antibiotic. A few small-scale academic clinical studies have supported these claims.
AVP-786 and CERC-301
And although the above drugs are currently sailing through pre-clinical and clinical trials, all is not rosy for all companies looking to mimic ketamine’s antidepressant effects. In November 2016, Cerecor’s CERC-301 hit a major hurdle when it failed to prove efficacy in a Phase II clinical trial against severe major depressive disorder.
CERC-301 is again being developed as an adjunctive therapy, and is a small molecule NR2B-specific antagonist of the NMDA receptor’s glutamate bonding site. The Baltimore, Maryland, US-based company have said there were some glimmers of hope in the trial findings and a decision will be made on the future of the molecule once the full trial results are assessed.
The NMDA receptor antagonist, AVP-786, being developed by Aliso Viejo, California, US-based firm Avanir recently met a similar fate in Phase II trials as an adjunctive therapy for major depressive disorder. ‘Since the study did not meet its primary endpoint, the company has decided not to pursue any additional studies,’ confirmed Avanir spokesperson Lez Cunningham.
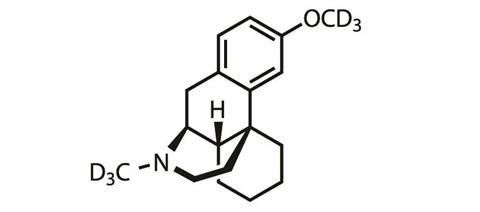
AVP-786 is a deuterated analogue of the cough suppressant dextromethorphan and given alongside a low dose of quinidine, that acts to slow down its metabolism (see here for more information).
One of the earlier molecules that failed to impress during Phase II trials was AstraZeneca’s Lanicemine, a small molecule NMDA receptor antagonist.
Mechanistic questions
The answer as to why some of these drugs are struggling to mimic the success of ketamine in trials could be that the NMDA receptor is the wrong target. ‘Ketamine is a very dirty drug – it goes a lot of places and it does a lot of things,’ explains Levine. ‘A recent paper in Nature showed that NMDA receptor antagonism was unnecessary and that ketamine exerts its antidepressant effects through AMPA [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid] receptor activation. This is a pre-clinical study within a mouse model, but still.’
‘Because ketamine is an established NMDA receptor antagonist, when its antidepressant effects were identified it was assumed that it was acting through that mechanism to exert its antidepressant effects,’ explains the Nature paper’s senior author Todd Gould, a neuroscientist at University of Maryland, Baltimore, US. However, when the early molecules that mimicked ketamine’s activity at the NMDA receptor failed to prove efficacy during clinical trials, it started to be suspected that the assumed mode of action might not be correct.
‘We, and others, started thinking about alternative mechanisms whereby ketamine may act,’ Gould says. His team explored whether ketamine’s metabolites were exerting its antidepressant effects, rather than ketamine itself. Ketamine has multiple metabolites, the most prevalent being (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine.
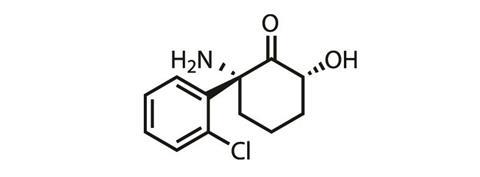
Gould’s team first checked that conversion to these hydroxynorketamines was necessary for the antidepressant effects on rodents to be seen. To do this they substituted a hydrogen for deuterium on the spot on ketamine’s structure where metabolism occurs – the new C–D bond is much stronger than the original C-H bond, meaning this atom substitution significantly slowed metabolism down. ‘What we found was that the deuterated version of ketamine did not exert the antidepressant effects that the ketamine did,’ he says. Thus proving metabolism was key to ketamine’s antidepressant properties.
A comparison of (2S,6S)-hydroxynorketamine to (2R,6R)-hydroxynorketamine found that the (2R,6R) has higher potency. ‘We also found that the (2R,6R)-hydroxynorketamine is not an antagonist of the NMDA receptor,’ Gould explains. The news then got even better: with tests finding that the molecule is very stable, readily enters the blood-brain barrier, has pretty good oral availability and, possibly most importantly, doesn’t induce any of the undesirable dissociative or addictive effects seem with ketamine.
‘The (2R,6R)-hydroxynorketamine has a lot of things you’d like to see in a drug candidate,’ explains Gould. His collaborators at the US National Institutes of Health’s National Center for Advancing Translational Sciences in Rockville, Maryland,US, are now pushing forward with exploring this possibility.
‘The work we’re undergoing right now,’ explains team member Patrick Morris, ‘is ensuring we can make this molecule in an efficient way so that it’s highly pure and in a format that can be easily administered to humans.’ Toxicology studies are also due to start imminently.
But what do Gould and Morris’s findings mean for the other groups with ketamine-mimics currently in the drug pipeline? Well, some of them are already on the case. ‘In mice, when we block the AMPA receptor our drug, AV-101, does not work, just like ketamine doesn’t work,’ explains VistaGen’s Smith. This research was done in collaboration with Gould. And the same has also been shown with rapastinel, he adds. ‘Our drug, ketamine, rapastinel, all seem to need the AMPA receptor activation to exert their rapid antidepressant effects.’
Our findings may have implications regarding the likely success of some the drug candidates, concedes Gould. ‘But the bottom line is for a drug it really doesn’t matter how it works, just that it does work and it is safe.’ And there are indeed many successful and long-approved drugs for which this is true.
Nina Notman is a science writer based near Baltimore, US












No comments yet