Two new reactions enable synthetic chemists to easily swap aromatic carbon atoms for nitrogen atoms, allowing the quick conversion of aromatic groups into heteroaromatics. The reactions will help drug discovery chemists quickly modify chemical structures to see how small structural variations influence the biological activity of compounds of interest. The researchers behind the work believe it will allow chemists to synthesise chemicals in a more intuitive way that closely matches the way the researchers actually conceptualise their target molecules.
We’ve totally subverted the stepwise approach
Mark Levin, University of Chicago
The field of ‘skeletal editing’ is a fast-developing area of synthetic chemistry, with many researchers devising new techniques to alter the structures of complex molecules in a variety of ways – whether by adding, deleting or swapping single atoms in a compound’s core motif. Mark Levin’s group at the University of Chicago, US, is particularly interested in editing techniques involving nitrogen atoms. In 2021, the group developed a method for deleting nitrogen atoms from organic structures, and last year the team revealed methods for deleting carbons from nitrogen containing aromatic groups and inserting nitrides into indenes to generate isoquinolines.
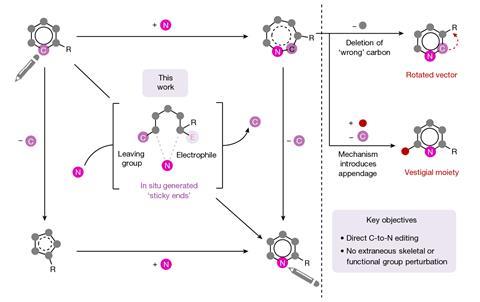
Now, the team has developed two new reactions for replacing aromatic carbon atoms and substituents connected to them, and swap them for a nitrogen. ‘This is something that conceptually medicinal chemists do all the time in the sense that they’ll be sitting there with a lead molecule that is not quite a drug, but it’s almost a drug, and they’ll think to themselves: “Well, we need to take this arene and replace it with a heteroarene where one of the carbons is a nitrogen”,’ says Levin. ‘And so, in their minds, or even on their ChemDraw screens, they will replace a carbon atom with a nitrogen. But until now, there haven’t really been methods to allow them to actually directly do that.’
Subverting conventions
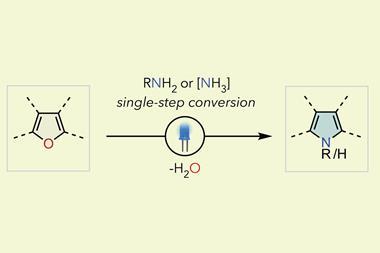
The first of Levin’s two new techniques works with simple aromatic compounds to which an azide group can be installed onto the carbon that the researchers ultimately wish to swap for a nitrogen.1 A two-step sequence carried out in a single flask first sees one nitrogen from the azide internalised into the ring via a photochemical reaction, before an oxidation reaction sees the carbon removed from the ring, leaving a pyridine product.
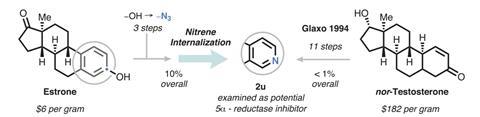
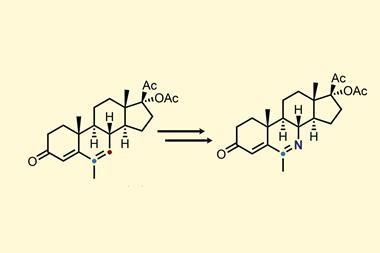
The team showed how the atom-swapping technique can be used in compounds of biological interest by converting the female sex hormone estrone into its pyridine analogue. Levin’s group’s strategy required just three steps to install the azide group followed by the nitrene internalisation process. Previously, making this compound would have required an 11-step synthesis from a starting material 30 times more expensive than estrone.
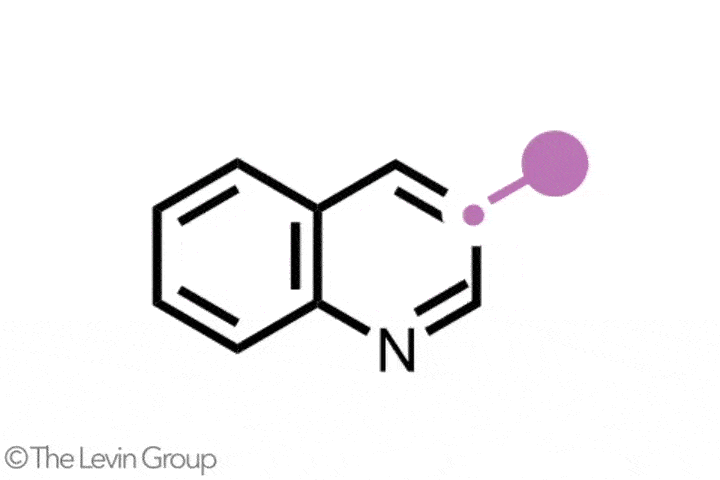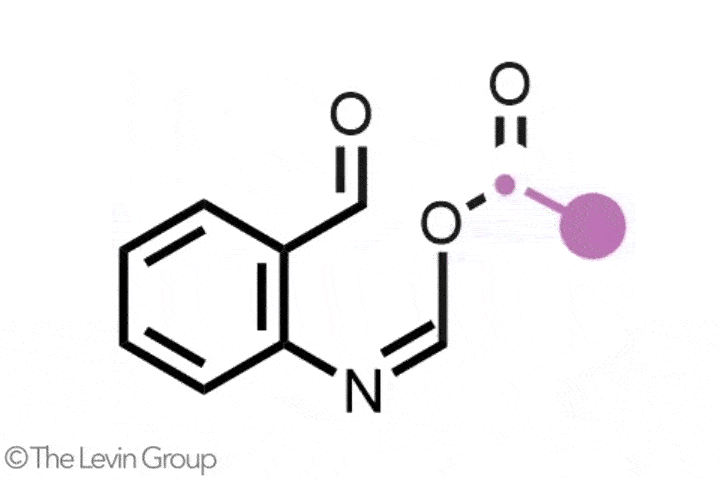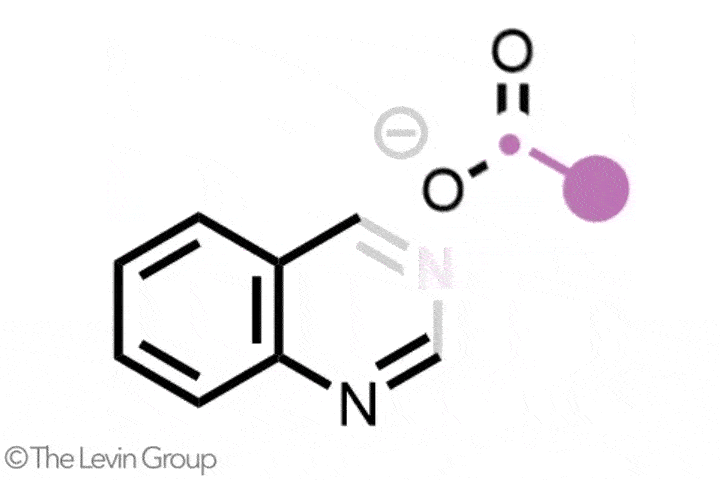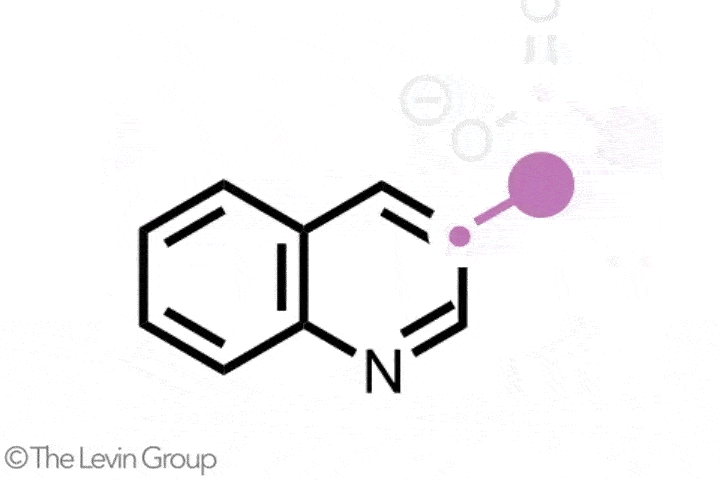
The second reaction takes quinolines – compounds featuring fused benzene and pyridine rings – and converts them into quinazolines, with a second nitrogen replacing one of the carbons of the original pyridine.2 This method differs from the first in that the reaction proceeds via a single step. Because the nitrogen insertion and carbon deletion take place at the same time, the reaction avoids any risk of the ring-opened intermediate rotating, which in some cases could affect the structure of the final product.
‘We’ve totally subverted the stepwise approach – it doesn’t go via an insertion–deletion sequence,’ explains Levin. ‘Instead, it cracks the ring open and it leaves behind reactive fragments that let you put it back together and kick out the carbon at the same time.’
‘Because the nitrogen is literally kicking the carbon out, there’s no opportunity for skeletal rotations,’ he adds.
Expanding chemistry
Richmond Sarpong, an expert in skeletal editing techniques based at the University of California, Berkeley, who wasn’t involved in the work, describes the two studies as a very important contribution to the area of atom swapping. ‘It shows that these reactions, which many chemists – especially those in the pharmaceutical, agrochemical and materials areas – had dreamed of, are possible,’ says Sarpong. ‘While it is true that in order to achieve the net transformation multiple manipulations are required, overall, these steps can all be streamlined as [Levin’s team has] described.’
‘The two transformations are also complementary and should find immediate use,’ he adds.
Levin notes that there are still limits to the types of substrates that the two reactions work with, but his group is determined to continue developing their methods and come up with new solutions to expand the chemistry further. ‘I’m not going to stop working on this problem until you can pick any aromatic carbon and any molecule, no matter how complex, and reliably turn it into a nitrogen – I really think this problem deserves that level of solution!’ he says.
For Levin, the biggest impact of these atom swapping reactions is the way they allow chemists to make molecules in the same way that they think about them – rather than having to design an entirely new synthesis just to make a seemingly small tweak to a compound’s structure, they can now simply swap atoms in a way that closely resembles the way they might do this on screen with molecule editing software.
‘There are probably examples where medicinal chemists didn’t even try to do the nitrogen replacement that they were envisioning, because it would have been too much work,’ notes Levin. ‘And that’s the case that I think is most interesting here … it’s the element here where the chemistry starts to behave the same way as the design process that I think is really enabling.’
References
1 T J Pearson et al, Science, 2023, DOI: 10.1126/science.adj5331
2 J Woo et al, Nature, 2023, DOI: 10.1038/s41586-023-06613-4











No comments yet