Novozymes is scouring the world for enzymes that make industrial processes more sustainable, as Mark Peplow discovers
At first glance, the lab looks like a Lilliputian laundry. Rows of miniature washing machines – some no bigger than a toaster – are whirring away, tumbling small swatches of cloth bearing a smorgasbord of stains.
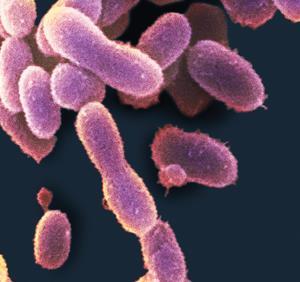
The detergent enzymes swishing around inside have come a long way to get here. Some were born inside microbes that make their home in the chilly Arctic, before being scooped up by bioprospectors and brought back to Bagsværd in Denmark. Here, they have been prodded, tweaked, and given a new host microbe – they have been transformed into super-enzymes.
Welcome to the detergent research centre at Novozymes, the world’s largest enzyme manufacturer. If you clean your clothes with the help of a biological washing powder, you are probably using their products. Mixed in with your laundry, their proteases slice through the proteins found in blood and sweat, lipases go to work on fatty stains, amylases tackle starchy residues, while cellulases trim dangling cellulose fibres from clothes, softening and brightening fabrics and helping to free trapped dirt particles.
Novozymes supplies about half of the global market for industrial enzymes, but enzymes can offer much more than a happy wash. Unlike some conventional chemical catalysts, they rarely get involved in side-reactions, making them extremely efficient in a range of industrial processes. ‘Enzymes are incredibly specific – you ask them to do one thing, and they do it perfectly,’ says Per Falholt, head of R&D at Novozymes. They can also be cheaply and easily brewed up in large fermentation vats. These credentials mean that Novozymes’ enzymes – and the microbes that make them – are attracting the attention of three other sectors: pharmaceuticals, agriculture and biofuels.
This expansion builds on enormous changes in industrial enzyme development over the past two decades, driven by genomics, protein chemistry and high-throughput screening. When the company was trying to develop improved washing powder enzymes in the early 1990s, recalls Falholt, it took 50 scientists a month to make a new enzyme for testing. ‘Right now, we make 10,000 a day,’ he says with delight. ‘The ability to create enzyme diversity in the lab has vastly increased, and so has the ability to screen and select the preferred diversity.’
But the company’s unusual ownership structure has also played a role in shaping its research. Novozymes’ majority shareholder is the Novo Nordisk Foundation, which uses the profits from its companies for scientific, social and humanitarian goals. As well as ploughing 15% of Novozymes’ revenue back into research and development, the foundation awarded €103 million (£77 million) in grants, fellowships and prizes in 2013, a figure that will rise to €200 million in 2018. Much of the scientific work they support in this way is focused on environmental sustainability and human health. ‘They’re doing some of the best stuff out there,’ says biochemist Gerald Joyce at the Scripps Research Institute in the US.
Let the hunt begin
Novozymes now boasts an annual revenue of about $2 billion (£1.3 billion) and a global staff of over 6000, including 1200 scientists, with more than 700 products used in 130 countries (see A brief history of Novozymes below). Its closest competitor is DuPont, with a 20% share of the global market.
Yet despite its technological capabilities, Novozymes’ enzyme discovery process often starts with a stroll in the countryside. ‘I like to walk in the woods with rain boots on as often as possible,’ says Mikako Sasa, a science manager and mycologist based at the company’s headquarters in Bagsværd, and one of the company’s intrepid enzyme hunters.

Fungi and bacteria are popular quarries because they are abundant and produce a huge array of different enzymes. ‘There’s no organic substrate on earth that cannot be degraded by either a fungus or a bacterium,’ says Sasa. Their genes are also conveniently compatible with the microbes commonly used for manufacturing enzymes in bulk, such as Bacillus bacteria or Aspergillus fungi.
Back in the lab, researchers grow the samples they collected using a range of different conditions. The fluid surrounding the microbes is then screened against around 30 different substrates that detect all the major types of enzymes. Any hits are probed with more complex assays to gauge the enzyme’s activity and its optimum working conditions.
Genomics has helped to speed up this process. After sequencing the genome of an organism, researchers look for areas of the code that are involved in making enzymes. Then the proteins produced by each organism are surveyed, using techniques such as mass spectrometry. This proteome can then be compared with the genome of the organism to identify the sequence that produces a particular enzyme.
Some of the most promising candidates then pass to protein engineering labs, where researchers try to improve their performance by tweaking their amino acid sequences. ‘There’s a healthy competition between our two groups,’ says Sara Landvik, Sasa’s fellow enzyme trapper. ‘For us, the challenge is to find something that is so good they don’t need to change it – for them, the challenge is to improve it.’
Celebrating diversity
One way to boost the activity of a natural enzyme is to introduce random mutations into the gene that encodes it. Occasionally, a variant will show enhanced activity, and high-throughput screening can search through a thousand variants for useful properties every second. But combing through every possible enzyme structure would be a fool’s errand. DNA encodes 20 different amino acids, and an enzyme might contain 350 amino acids – so the number of possible combinations probably exceeds the number of molecules in the known universe, reckons Falholt.
So Novozymes’ protein engineers often use a more strategic approach: making specific changes to an enzyme’s active site. Sasa likens the process to running a marathon: ‘Nature has done the 40km – they can do the last two.’ Once the activity is as good as can be, it then passes over to the unit that works with customers to match enzymes to their needs.
With the advent of synthetic biology, one might think that researchers are on the verge of being able to design and build enzymes from scratch. ‘But I’m a little bit skeptical of synthetic biology, because you cannot beat four billion years of evolution,’ says Falholt. And why bother when nature has plenty more surprises waiting to be uncovered. Each bacterium typically hosts around 3000 different enzymes, while fungi have up to 6000, and Falholt estimates that only about 3% of all natural enzymes are known.

Novozymes already has roughly 50,000 bacteria and fungi in its collection, which dates back over 60 years. ‘But it’s not a numbers game, it’s about diversity,’ says Sasa. ‘It’s easy to get high numbers – I can take a soil sample in our back yard and isolate thousands of strains. We want as many different types of organism as possible – strains that like low pH, high pH, low temperature, high temperature, degrading different things, because you never know what our customers will want.’
If the goal is a washing powder that works effectively at 15°C, it needs a protease that is active at low temperatures – so the enzyme hunters head for the Arctic. ‘The tendency now is that we’re going to cold places, because the colder the application can be done, the more energy you are saving,’ says Sasa.
Sometimes, though, amazing enzymes can turn up closer to home. ‘I often go to cemeteries, they’re a very nice place to look for fungi,’ says Sasa. ‘The grounds are fertilised and the soil is quite alkaline and nutrient rich, so you get interesting strains.’
One of the companies most successful proteases – launched in 1974 as Esperase, a detergent enzyme – was discovered in the late 1960s in the Assistens Cemetery in Copenhagen, final resting place of philosopher Søren Kierkegaard and physicist Niels Bohr. Esperase is very tolerant to high pH, explains Falholt. ‘It’s the most aggressive protease we have; it’ll take the skin off your fingers in less than a minute.’
Bacteria and fungi are not the only sources of new enzymes, however. Novozymes has been involved with the five-year Novenia academic-industry collaboration, which ran until the end of 2014. The project hunted for protease enzymes in spiders, snakes and carnivorous plants, as well as extremophile microbes, and has identified the genetic sequences of hundreds of new enzymes.
‘We know that extremely aggressive digestion processes are going on in these species that are very different from those in humans,’ says Kristian Wejse Sanggaard, a protein chemist at Aarhus University in Denmark, who was involved in Novenia. In the digestive juices of the Venus flytrap, Novenia researchers discovered a chitinase that can chomp through tough insect chitin. But the biggest surprise was in spiders, which rely heavily on metalloprotein enzymes during digestion. ‘Before, the digestion process in spiders was completely unknown,’ says Sanggaard.
DuPont, one of the project partners, is now building on these exotic discoveries, while Novozymes is following up on some of the enzymes derived from the extremophile microbes, including one found in the cold and highly alkaline bottom-waters of a Greenland fjord.
Down on the farm
Novozymes’ fastest growing sector is biofuels, which saw a 19% increase in sales last year. The company is the world’s largest supplier of enzymes to make bioethanol from the sugars in crops such as sugar cane and maize. Amylases and saccharification enzymes help to break down the plant’s starch and glycogen into sugars; other enzymes reduce the viscosity of the mash, to improve its flow.
But this form of biofuel production offers relatively small savings in carbon emissions, and has also been blamed for driving up food prices. One major problem is that most of the biofuel crop is wasted, because the cellulose and hemicellulose molecules in the leaves and stalks are hard to break down into fermentable sugars.
Novozymes’ solution is a cocktail of cellulase enzymes, dubbed Cellic, which can chew up these tough molecules. In 2013, the world’s first full-scale cellulosic biofuel production facility opened in Italy, using Novozymes’ enzymes to break down wheat straw and other plant fibres. In December 2014, a similar facility came online in Brazil that uses sugarcane waste as its feedstock, while planned facilities in the US will digest a wild reed and even household garbage. Novozymes reckons that by 2017 it will be supplying enzymes to 15 advanced bioethanol plants around the world.
Meanwhile, the company is expanding its agricultural products business. It began a $300 million collaboration with Monsanto last year called the BioAg Alliance, which aims to develop microbes to improve crop yields and ward off pests and diseases. Novozymes is setting up a bioagriculture research centre in North Carolina to support the work.
Last year, the alliance tested hundreds of microbial strains in more than 170,000 crop trials. On 8 January, the alliance reported some of these strains could increase maize and soy yields by roughly 2% and 4% respectively.
A brief history of Novozymes
Novozymes traces its history back to 1925, when brothers Harald and Thorvald Pedersen founded Novo Terapeutisk Laboratorium to manufacture insulin. The source of that insulin – the pancreas of cattle – also contained useful enzymes, which the company discovered how to isolate in the early 1940s.
Novo’s first enzyme product, trypsin, was launched in 1945 as a pre-treatment stage in leather tanning. But extraction from the pancreas was not a particularly efficient way to produce enzymes, so in the 1950s the company started to use microbial fermentation to make amylases, which could remove starch from fabrics. The brothers established the Novo Foundation in 1951, and its successor, Novo A/S, a subsidiary of the Novo Nordisk Foundation, is still the majority shareholder in Novozymes.
In 1963, Novo launched Alcalase, the first detergent enzyme produced by fermenting microbes. But the fight against stains continued, and in 1987 the company launched Lipolase to bust fat stains, the first enzyme produced from genetically-modified microbes. After a series of mergers and de-mergers, the company’s enzyme business was hived off as Novozymes in 2000.
Long-life drugs
Over the past decade, Novozymes has also developed products for the pharmaceutical industry, particularly recombinant human serum albumin (HSA). HSA is the most common protein in blood, where it transports hormones, fatty acids and other biomolecules. It has a very long half-life in the body – about 19 days – because it binds to cell surface receptors that help to protect it from degradation inside the cell. That makes HSA useful in medicines and vaccines, particularly for proteins and peptides used to treat chronic conditions, because it can help to ensure that they stay in the body for longer, reducing the number of doses needed.
Until recently HSA was largely gathered from donated human blood, which could be inconvenient, expensive and prone to viral contamination. But in 1999, Delta Biotechnology – a company set up by Bass Brewery in Nottingham, UK – began manufacturing HSA using a modified strain of the brewer’s yeast Saccharomyces cerevisiae. In 2005, it won approval from the US Food and Drug Administration for its Recombumin HSA to be used in Merck’s MMR-II vaccine. Novozymes bought Delta the following year, forming Novozymes Biopharma.

In 2009, Novozymes’ researchers realised that if HSA bound more strongly to cell receptors, it has an even longer half-life. Modifying the yeast – and the enzymes that it uses to make HSA – meant that they could tweak the protein’s structure to control how long it stayed in the body. The result is Veltis, a cocktail of natural and engineered human albumins that can help therapeutic proteins linger inside patients for just the right amount of time.
Veltis saw its first commercial breakthrough last year as a component in Eperzan (albiglutide), a type 2 diabetes treatment produced by GlaxoSmithKline. By extending the half-life of the drug, patients need only one injection a week, rather than one every day, and future developments could reduce that to monthly injections. Novozymes is now working with other pharmaceutical companies to apply the technology to other drugs.
Seeking sustainability
Looking to the future, Falholt says that Novozymes hopes to cut the time from bench to market by developing ever-faster screening processes that can pick winners from mere picolitres of enzyme solutions. Improvements in protein crystallography and structure prediction would also help refine their activity.
But the most significant shift could involve how novel enzymes are used by industry. Most of Novozymes’ enzymes are currently sold as separate ingredients that are added to batch processes. Now, though, there is a growing emphasis on developing microbes – such as the HSA-secreting yeast – that can live within industrial production processes, continuously producing the enzymes that are needed. After all, says Joyce, ‘you don’t take the enzymes out of yeast to brew beer’.
All these research goals rest on three core principles, says Falholt: ‘Reduce waste, save energy, be more sustainable.’ And he is convinced that enzymes – and the microbes that make them – will become ever more vital to our lives in the coming years. ‘If we don’t start using biological processes to a much higher extent, I think we’ll be in trouble, because the way we use resources today is unsustainable.’
Mark Peplow is a science journalist based in Cambridge, UK











No comments yet