All hydrogen bonding articles
-
 Research
ResearchStiffest organic crystals reported to date discovered in well-known material
Mucic acid crystals owe their rigidity to a dense network of intermolecular hydrogen bonds
-
 Research
ResearchWater squeezed into 2D channels conducts electricity 100,000 times better
Network of quasi-2D hydrogen bonding may be responsible for effect
-
 Research
ResearchFar more drugs could be taken orally by formulating them with silica nanoparticles
Many more medicines could be made water soluble making taking them easier for patients
-
 Research
ResearchNew spectroscopy method maps out water’s hydrogen-bonded network
Technique can be used to study other liquids and amorphous materials
-
 Opinion
OpinionA high-pressure insight into the structure of water
The hydrogen-bonded network in liquid water resists compression; density increases instead arise from molecules moving into voids
-
 News
NewsBeyond hydrogen bonding: new definitions for secondary bonding interactions to end confusion
The 20-year struggle to define secondary bonding interactions
-
 Research
ResearchHydrogen bonding helps to maintain tear film stability
Research uncovers how hydroxyl-containing solutes delay eye dewetting process
-
 Feature
FeatureReaching into the non-covalent toolbox
Alongside supramolecular stalwarts, budding bonding forms are vying to be valuable, finds Andy Extance
-
 Research
ResearchVan der Waals crust behind simple parameter that can describe chemical bonds
Penetration index provides a fresh perspective on two-atom interactions
-
 Research
ResearchComputational study says polonium can form hydrogen bonds
Bonds driven by relativistic effects, rather than electronegativity differences
-

-
 Research
ResearchQuantum nature of hydrogen bonds observed in acid–base complex
Spectroscopic evidence of proton delocalisation could change the way we approach acid–base chemistry
-
 Research
ResearchUnusual hydrogen bonds found in proteins help them bind their targets
Weak interactions between hydrogen and carbon atoms have synthetic chemistry implications
-
 Opinion
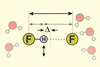
OpinionWhen does a hydrogen bond become a covalent bond?
Ultrafast infrared spectroscopy probes the character of the short, strong bonds in HF2–
-
 Research
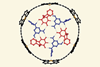
ResearchIntricate supramolecular rosette demonstrates power of cooperative interactions
Rosette assembles inside porphyrin nanoring when all components are present
-
 Research
ResearchHydrogen bonds join forces to maximise water–water interaction
Cooperative environment encourages stronger chemistry
-
 Research
ResearchSimulation says supercritical water has no hydrogen bonds
Computational approach seeks to clarify bonding confusion
-
 Research
ResearchHydrogen bond imparts more stability on transition state than expected
Study reveals importance of repulsive interactions
-
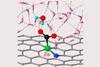 Research
ResearchHydrogen bonds are single atom nickel’s secret for carbon dioxide reduction
Computational calculations show that hydrogen atoms and charge capacity are behind nickel’s high activity and selectivity in electrochemical carbon dioxide reduction
-
 Opinion
OpinionWill computers ever discover drugs from scratch?
With enough understanding and computing power, it should be possible, but will it happen?